| BYOSENS LYTE96,多模式微孔板检测仪免标记系统,多模式微孔板检测仪,基于Corning Epic 系统光学免标记技术的台式微孔板检测仪 |
| 型号:BYOSENS LYTE96 |
| 价格:请致电:010-67529703 |
| 品牌:byosens |
BYOSENS LYTE96台便携式wu标记酶标仪(便携微孔板检测器)
基于Corning Epic 系统的、使用光学免标记技术的台式微孔板检测仪
BYOSENS LYTE96wu标记便携式wu标记酶标仪(便携微孔板检测器)系统介绍
lyte96wu标记便携式wu标记酶标仪(便携微孔板检测器)是基于康宁Epic系统设计的,可进行一系列细胞内试验的96孔微孔板读出设备。lyte96将wu线连接和集成电池结合放置到一个紧凑的结构中,使得它方便移动和易于整合进液体处理系统。主要是对系列广泛的生物反应进行检测,如信号转导、细胞凋亡、细胞毒素,贴壁、增殖和扩散等。 lyte96wu标记便携式wu标记酶标仪(便携微孔板检测器)的工作原理是基于折射波导光栅光学生物传感器。传感器结构由一个三层系统:玻璃基板、薄膜光波导薄膜与光栅结构,和细胞/生物分子层。当宽谱带光照射时,生物传感器反映光的te定波长是接近传感器表面折射率的灵敏函数。通过 Epic系统测量细胞内的粘合物事件或细胞内蛋白质运动引起反射光的波长偏移。形成一系列波长偏移、波长、强度、时间之间的函数来进行分析。 lyte96wu标记便携式wu标记酶标仪(便携微孔板检测器)的势: 移动性: lyte96创新设计之处是给使用者带来了j大的灵活性。紧凑的结构结合了wu线连接和集成的电池使lyte96方便移动。这使得它对于研究人员和开发人员来说成为一个完美的分析工具。 易用性: lyte96简化了研发实验室中的过程。实验开始时不需要复杂的预置,直观辅助的软件保证了高水平的易用性。由于专利技术体系,lyte96几乎是免费维护。 数据分析:根据已建立的康宁Epic系统,高敏性的lyte96可进行宽光谱的细胞内试验,从开始试验到几天的时间都可以提供实时数据以便研究。
图1. 莱te96wu标记便携式wu标记酶标仪(便携微孔板检测器)
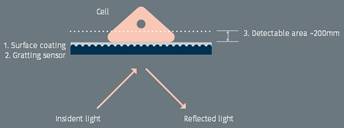
图2.测量原理示意图
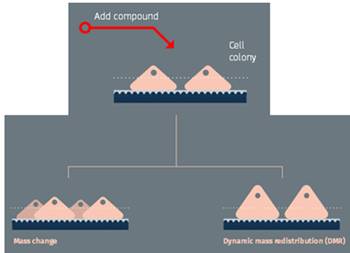
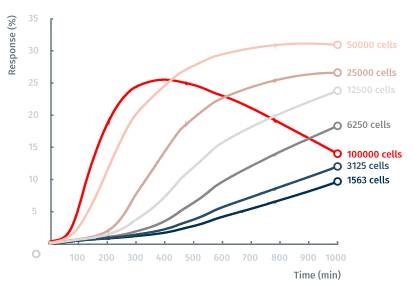
例1:在增殖试验中,用lyte96实时监测细胞数量,发现细胞数目和传感器表面的质量是成正比的。微孔板和lyte96放置在加湿的培养箱内通过蓝牙wu线连接电脑。经典增殖试验中,A431细胞加入到孔中,记录37˚C的细胞生长。
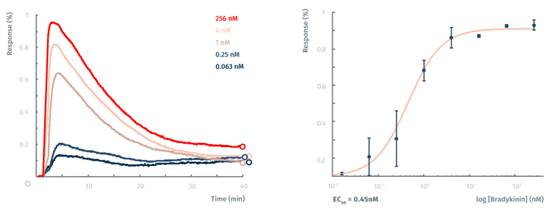
像许多其他的信号检测,GPCR测定动态质量再分配过程中(DMR)是由lyte96wu标记传感器测定的。和A431细胞缓激肽试验一样,这个试验是在室温下进行。得到的EC50为0.45 nm,这类似于从文献的结果。 参考文献: Nazirizadeh, Y. et al. Intensity interrogation near cutoff resonance for label-free cellular profiling. Sci. Rep. 6, 24685 (2016).
French, J. B. et al. Spatial colocalization and functional link of purinosomes with mitochondria. Science 351, 733 (2016).
Camp, N. D. et al. Dynamic mass redistribution reveals diverging importance of PDZ-ligands for G protein-coupled receptor pharmacodynamics. Pharmacological. Research, 105, 13-21 (2016).
Klein, A. B., Nittegaard-Nielsen, M., Christensen, J. T., Al-Khawaja, A., & Wellendorph, P. Demonstration of the dynamic mass redistribution label-free technology as a useful cell-based pharmacological assay for endogenously expressed GABAA receptors. Med. Chem. Commun., 7, 426–432 (2016).
Klepac, K. et al. The Gq signalling pathway inhibits brown and beige adipose tissue.Nat. Commun. 7, 10895 (2016).
Hamamoto, A., Kobayashi, Y. & Saito, Y. Identification of amino acids that are selectively involved in Gi/o activation by rat melanin-concentrating hormone receptor 1. Cell. Signal. 27, 818–827 (2015).
Navarro, G. et al. Orexin – Corticotropin-Releasing Factor Receptor Heteromers in the Ventral Tegmental Area as Targets for Cocaine. J. Neurosci. 35, 6639–6653 (2015).
Wang, J. et al. RSC Advances danshen using a label-free cell phenotypic assay. RSC Adv. 5, 25768–25776 (2015).
Rex, E. B. et al. Phenotypic Approaches to Identify Inhibitors of B Cell Activation. J. Biomol. Screen. 20, 876–886 (2015).
Vinals, X. et al. Cognitive Impairment Induced by Delta9- tetrahydrocannabinol Occurs through Heteromers between Cannabinoid CB 1 and Serotonin 5-HT 2A Receptors. PLOS Biol., e1002194 (2015).
Fjellström, O. et al. Novel Zn 2+ Modulated GPR39 Receptor Agonists Do Not Drive Acute Insulin Secretion in Rodents. PLoS One, 0145849 (2015).
Shridhar, N. et al. The experimental power of FR900359 to study Gq-regulated biological processes. Nat. Commun. 6, 10156 (2015).
Marada, S. et al. Functional Divergence in the Role of N-Linked Glycosylation in Smoothened Signaling. PLOS Genet., 1005473 (2015).
Brust, T. F., Hayes, M. P., Roman, D. L. & Watts, V. J. New functional activity of aripiprazole revealed: robust antagonism of D2 dopamine receptor-stimulated Gβγ signaling. Biochem Pharmacol. 93, 85–91 (2015).
Camp, N. D. et al. Individual protomers of a G protein-coupled receptor dimer integrate distinct functional modules. Cell Discov. 1, 15011 (2015).
Beckert, U. et al. Biochemical and Biophysical Research Communications cNMP-AMs mimic and dissect bacterial nucleotidyl cyclase toxin effects. Biochem. Biophys. Res. Commun. 451, 497–502 (2014).
Otte, M. et al. CXCL14 is no direct modulator of CXCR4. FEBS Lett. 588, 4769–4775 (2014).
Liebscher, I. et al. A Tethered Agonist within the Ectodomain Activates the Adhesion G Protein-Coupled Receptors GPR126 and GPR133. Cell Rep. 9, 2018–2026 (2014).
Fang, Y. Label-Free Cell Phenotypic Drug Discovery. Comb. Chem. High Throughput Screen. 17, 566–578 (2014).
Fang, Y. Label-free drug discovery. Front. Pharmacol. 5, 1–8 (2014).
Febles, N. K., Ferrie, A. M. & Fang, Y. Label-Free Single Cell Kinetics of the Invasion of Spheroidal Colon Cancer Cells through 3D Matrigel. Anal. Chem. 86, 8842–8849 (2014).
Lee, M. Y. et al. A Comparison of Assay Performance Between the Calcium Mobilization and the Dynamic Mass Redistribution Technologies for the Human Urotensin Receptor. Assay Drug Dev. Technol. 12, 361–368 (2014).
Balenga, N. A. et al. Heteromerization of GPR55 and cannabinoid CB2 receptors modulates signalling. Br. J. Pharmacol. 171, 5387–5406 (2014).
Carter, R. L. et al. Dynamic mass redistribution analysis of endogenous b -adrenergic receptor signaling in neonatal rat cardiac fibroblasts. Pharma. Res. Per.2, 1–16 (2014).
Teutsch, C. et al. Detection of free fatty acid receptor 1 expression : the critical role of negative and positive controls. Diabetologia 57, 776–780 (2014).
Meister, J. et al. The G Protein-coupled Receptor P2Y 14 Influences Insulin Release and Smooth Muscle Function in Mice. J. Biol. Chem. 289, 23353–23366 (2014).
Andradas, C. et al. Targeting CB 2 -GPR55 Receptor Heteromers Modulates Cancer Cell Signaling. J. Biol. Chem. 289, 21960–21972 (2014).
Schmitz, J. et al. Dualsteric Muscarinic Antagonists − Orthosteric Binding Pose Controls Allosteric Subtype Selectivity. J. Med. Chem. 57, 6739–6750 (2014).
Mackenzie, A. E. et al. The Antiallergic Mast Cell Stabilizers Lodoxamide and Bufrolin as the First High and Equipotent Agonists of Human and Rat GPR35. Mol. Pharmacol.85, 91–104 (2014).
Chen, X. et al. Rational Design of Partial Agonists for the Muscarinic M1 Acetylcholine Receptor. J. Med. Chem. 58, 560–576 (2014).
Ferrie, A. M., Zaytseva, N. & Fang, Y. Divergent Label-free Cell Phenotypic Overexpressed b2-Adrenergic Receptors. Sci. Rep. 4, 3828 (2014).
Orgovan, N. et al. Dependence of cancer cell adhesion kinetics on integrin ligand surface density measured by a high-throughput label-free resonant waveguide grating biosensor. Sci. Rep. 4, 4034 (2014).
Sun, H. et al. Label-free cell phenotypic profiling decodes the composition and signaling of an endogenous ATP-sensitive potassium channel. Sci. Rep. 4, 4934 (2014).
Sundström, L., Greasley, P. J., Engberg, S., Wallander, M. & Ryberg, E. Succinate receptor GPR91 , a G ai coupled receptor that increases intracellular calcium concentrations through PLC b. FEBS Lett. 587, 2399–2404 (2013).
Fang, Y. Troubleshooting and deconvoluting label-free cell phenotypic assays in drug discovery. J. Pharmacol. Toxicol. Methods 67, 69–81 (2013).
Ahmedat, A. S. et al. Pro-fibrotic processes in human lung fibroblasts are driven by an autocrine / paracrine endothelinergic system. Br. J. Pharmacol. 168, 471–487 (2013).
Morse, M., Sun, H., Tran, E., Levenson, R. & Fang, Y. Label-free integrative pharmacology on-target of opioid ligands at the opioid receptor family. BMC Pharmacol. Toxicol. 14, 1–18 (2013).
Online, V. A., Ferrie, A. M., Wang, C. & Fang, Y. Integrative Biology identifies an intracellular signalling wave mediated through the b2-adrenergic receptor. Integr. Biol. 5, 1253–1261 (2013).
Christiansen, E. et al. Discovery of a Potent and Selective Free Fatty Acid Receptor 1 Agonist with Low Lipophilicity and High Oral Bioavailability. J. Med. Chem. 56, 982–992 (2013).
Hennig, D. et al. Novel Insights Into Appropriate Encapsulation Methods for Bioactive Compounds Into Polymers: A Study With Peptides and HDAC Inhibitors.Macromol. Biosci. 1–12 (2013).
Deng, H., Sun, H. & Fang, Y. Label-free cell phenotypic assessment of the biased agonism and efficacy of agonists at the endogenous muscarinic M3 receptors. J. Pharmacol. Toxicol. Methods 68, 1–24 (2013).
Zaytseva, N. et al. Resonant waveguide grating biosensor-enabled label-free and fluorescence detection of cell adhesion. Sens. Actuators B Chem. 1–17 (2013).
Zhao, H., French, J. B., Fang, Y. & Benkovic, S. J. The purinosome, a multi-protein complex involved in the de novo biosynthesis of purines in humans. Chem. Commun. (Camb). 49, 1–17 (2013).
Cho, Y. & Baldán, A. Quest for New Biomarkers in Atherosclerosis. Mo. Med. 110, 325–330 (2013).
Hennen, S. et al. Decoding Signaling and Function of the Orphan G Protein– Coupled Receptor GPR17 with a Small-Molecule Agonist. Sci. Signal. 6, 1–33 (2013).
Deng, H. & Fang, Y. The Three Catecholics Benserazide, Catechol and Pyrogallol are GPR35 Agonists. Pharmaceuticals 6, 500–509 (2013).
Deng, H., Wang, C. & Fang, Y. Label-free cell phenotypic assessment of the molecular mechanism of action of epidermal growth factor receptor inhibitors. RSC Adv. 3, 10370–10378 (2013).
Schrage, R. et al. Agonists with supraphysiological efficacy at the muscarinic M2 ACh receptor. Br. J. Pharmacol. 169, 357–370 (2013). |