ScreenCell 稀有循环细胞分离试剂盒,ScreenCell MB KIT分子生物套装,ScreenCell CC KIT细胞培养套装,ScreenCell Cyto KIT细胞学套装
型号:ScreenCell Cyto KIT,ScreenCell MB KIT,ScreenCell CC KIT(CC kit已停产)
联系人:李先生
联系电话:18618101725
品牌:screencell
现货促销
ScreenCell 稀有循环细胞分离试剂盒——
ScreenCell® MB KIT分子生物套装,ScreenCell® CC KIT细胞培养套装,ScreenCell® Cyto KIT细胞学套装
ScreenCell size-exclusion device features
|
 |
 ScreenCell® Isolation Devices
ScreenCell® Isolation Devices

Info+

Info+

Info+
 ScreenCell® Dilution Buffers
ScreenCell® Dilution Buffers
ScreenCell® Fixed Cells (FC2) Dilution Buffer
The ScreenCell® FC2 dilution buffer is essentially for pathocytological studies, including cell enumeration, cytology, immunochemistry and FISH assays (accessorily, molecular biology applications are possible through laser microdissection). It allows lysis of RBCs before filtration while preserving other cells. This buffer is for cytology, immunochemistry and molecular biology applications on fixed cells.
ScreenCell® Live Cells (LC) Dilution Buffer
The ScreenCell® CC dilution buffer is for isolation of live cells for cell culture directly on the filter after filtration through: (i) the ScreenCell® CC filtration device for further cytological applications; (ii) the ScreenCell® MB filtration device for further molecular biology (DNA/RNA extraction) applications. It allows a mild lysis of red blood cells (RBCs) before filtration while leaving other cells alive.
产品名称 |
状态 |
ScreenCell® MB KIT分子生物套装3支/盒 |
现货促销 |
ScreenCell® CC KIT细胞培养套装3支/盒 |
一应 |
ScreenCell® Cyto KIT细胞学套装4支/盒 |
现货促销 |
循环肿瘤细胞(CTCs)的分选与检测已经成为肿瘤的早期诊断,化疗药物效果评估,个体化的治疗方案提供的重要依据,目前国内外也相继出现了各种分选检测方法,可惜的是每种方法几乎都需要专门而昂贵的仪器设备,同时,每次检测实验也需要消耗昂贵的试剂耗材成本。为此,法国ScreenCell公司研发了在实验条件和价格上都能够普及应用的循环肿瘤细胞分选试剂盒。
世联博研(北京)科技有限公司的法国ScreenCell循环肿瘤细胞(CTCs)分选产品是目前国际同类产品中性价比高的产品,在欧美CTCs科研领域已被广泛使用。wu需要购置专用设备,客户在自己的实验室中,利用常规仪器即可随时完成方便、准确、高效、快速的CTCs检测,并且可以对分选出来的CTCs细胞进行后续的培养、染色等处理。
自即日起至2014年6月30日止,世联博研(北京)科技有限公司开展法国ScreenCell循环肿瘤细胞(CTCs)分选套装惠促销活动,内容如下:




产品名称: ScreenCell稀有循环细胞分离试剂盒
产品型号: ScreenCell
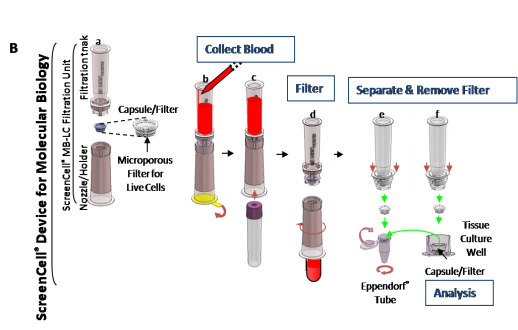
ScreenCell稀有循环细胞分离试剂盒是一项创新、简单、wu创的技术,主要用于从血分离循环稀有细胞或从任何生物液体中分离潜在的非典型细胞。能够对循环肿瘤细胞(CTCs)、循环肿瘤微血栓(CTMs)进行分离;并通过对孕妇外周血中循环胎儿细胞(CFCs)的分离,开发出wu创式产前诊断新方法。
ScreenCell稀有循环细胞分离试剂盒
新的稀有循环细胞的分离技术
ScreenCell稀有循环细胞分离试剂盒采用创新的技术,对外周血循环稀有细胞进行便捷、快
速、高灵敏度地分离。
技术——wu创技术进行稀有循环细胞分离:
ScreenCell®是一项创新、简单、wu创的技术,主要用于从血分离循环稀有细胞或从任何生物液体中分离潜在的非典型细胞。
ScreenCell®能够对循环肿瘤细胞(CTCs)、循环肿瘤微血栓(CTMs)进行分离;并通过对孕妇外周血中循环胎儿细胞(CFCs)的分离,开发出wu创式产前诊断新方法。
ScreenCell是目前的多功能稀有细胞分离技术:
wu需任何仪器设备,节省仪器的采购运行成本;
设计用于标准化的IVD分析平台;
分离过程仅需3分钟;
分离对象:固定化细胞、活细胞、残留液
不依赖与EpCAM
应用文献
Publications
2019October, 4th
Expression Profiling of Circulating Tumor Cells in Pancreatic Ductal Adenocarcinoma Patients: Biomarkers Predicting Overall Survival
Front Oncol. 2019 Sep 10;9:874. doi: 10.3389/fonc.2019.00874. eCollection 2019.
Amantini C1, Morelli MB1,2, Nabissi M2, Piva F3, Marinelli O1,2, Maggi F4, Bianchi F5, Bittoni A5, Berardi R5, Giampieri R5, Santoni G2.
Abstract
The interest in liquid biopsy is growing because it could represent a non-invasive prognostic or predictive tool for clinical outcome in patients with pancreatic ductal adenocarcinoma (PDAC), an aggressive and lethal disease. In this pilot study, circulating tumor cells (CTCs), CD16 positive atypical CTCs, and CTC clusters were captured and characterized in the blood of patients with PDAC before and after palliative first line chemotherapy by ScreenCell device, immunohistochemistry, and confocal microscopy analysis. Gene profiles were performed by digital droplet PCR in isolated CTCs, five primary PDAC tissues, and three different batches of RNA from normal human pancreatic tissue. Welsh’s t-test, Kaplan-Meier survival, and Univariate Cox regression analyses have been performed. Statistical analysis revealed that the presence of high CTC number in blood is a prognostic factor for poor overall survival and progression free survival in advanced PDAC patients, before and after first line chemotherapy. Furthermore, untreated PDAC patients with CTCs, characterized by high ALCAM, POU5F1B, and SMO mRNAs expression, have shorter progression free survival and overall survival compared with patients expressing the same biomarkers at low levels. Finally, high SHH mRNA levels are negatively associated to progression free survival, whereas high vimentin mRNA levels are correlated with the most favorable prognosis. By hierarchical clustering and correlation index analysis, two cluster gene signatures were identified in CTCs: the first, with high expression of VEGFA, NOTCH1, EPCAM, IHH, is the signature of PDAC patients before chemotherapy, whereas the second, with an enrichment in the expression of CD44, ALCAM, and POU5F1B stemness and pluripotency genes, is reported after palliative chemotherapy. Overall our data support the clinic value of the identification of CTC’s specific biomarkers to improve the prognosis and the therapy in advanced PDAC patients.
KEYWORDS:
atypical CTC; circulating tumor cells; digital droplet PCR; gene signature; overall survival; pancreatic cancerPMID: 31552188 PMCID: PMC6746928 DOI: 10.3389/fonc.2019.00874
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6746928/pdf/fonc-09-00874.pdf
Author information
1School of Biosciences and Veterinary Medicine, University of Camerino, Camerino, Italy.2School of Pharmacy, Experimental Medicine Section, University of Camerino, Camerino, Italy.3Department of Specialistic Clinical and Odontostomatological Sciences, Polytechnic University of Marche, Ancona, Italy.4Department of Molecular Medicine, Sapienza University of Rome, Rome, Italy.5Oncology Clinic, AOU Ospedali Riuniti, Polytechnic University of Marche, Ancona, Italy.
August, 5th
Circulating Tumor Cells in Right- and Left-Sided Colorectal Cancer
Nicolazzo C1, Raimondi C2, Gradilone A1, Emiliani A2, Zeuner A3, Francescangeli F3, Belardinilli F1, Seminara P2, Loreni F1, Magri V4, Tomao S2, Gazzaniga P5.
1 Department of Molecular Medicine, Circulating tumor cells Unit, Sapienza University of Rome, 00161 Rome, Italy.
2 Department of Radiological, Oncological and Pathological Sciences, Division of Medical Oncology, Sapienza University of Rome, 00161 Rome, Italy.
3 Department of Hematology, Oncology and Molecular Medicine, Istituto Superiore di Sanità, 00161 Rome, Italy.
4 Department of Surgical Sciences, Sapienza University of Rome, 00161 Rome, Italy.
5 Department of Molecular Medicine, Circulating tumor cells Unit, Sapienza University of Rome, 00161 Rome, Italy. paola.gazzaniga@uniroma1.it.
Abstract
Molecular alterations are not randomly distributed in colorectal cancer (CRC), but rather clustered on the basis of primary tumor location underlying the importance of colorectal cancer sidedness. We aimed to investigate whether circulating tumor cells (CTC) characterization might help clarify how different the patterns of dissemination might be relative to the behavior of left- (LCC) compared to right-sided (RCC) cancers. We retrospectively analyzed patients with metastatic CRC who had undergone standard baseline CTC evaluation before starting any first-line systemic treatment. Enumeration of CTC in left- and right-sided tumors were compared. The highest prognostic impact was exerted by CTC in left-sided primary cancer patients, even though the lowest median number of cells was detected in this subgroup of patients. CTC exhibit phenotypic heterogeneity, with a predominant mesenchymal phenotype found in CTC from distal compared to proximal primary tumors. Most CTC in RCC patients exhibited an apoptotic pattern. CTC in left-sided colon cancer patients exhibit a predominant mesenchymal phenotype. This might imply a substantial difference in the biology of proximal and distal cancers, associated with different patterns of tumor cells dissemination. The poor prognosis of right-sided CRC is not determined by the hematogenous dissemination of tumor cells, which appears to be predominantly a passive shedding of non-viable cells. Conversely, the subgroup of poor-prognosis left-sided CRC is reliably identified by the presence of mesenchymal CTC.
KEYWORDS:
CellSearch®; ScreenCell®; circulating tumor cells; colorectal cancer; epithelial-mesenchymal transition; prognosis; sidednessPMID: 31344798 DOI: 10.3390/cancers11081042
March, 21st
Non-Metastatic Esophageal Adenocarcinoma: Circulating Tumor Cells in the Course of Multimodal Tumor Treatment
February, 22nd
Liquid Biopsy in Rare Cancers: Lessons from Hemangiopericytoma
Abstract
Hemangiopericytoma (HPT) is a rare mesenchymal tumor of fibroblastic type and for its rarity is poorly studied. The most common sites of metastatic disease in patients with intracranial HPT are the bone, liver, and lung, suggestive for an hematogenous dissemination; for this reason, we investigated, for the first time, the presence of circulating tumor cells (CTCs) in hemangiopericytoma patient by CellSearch® and SceenCell® devices. Peripheral blood samples were drawn and processed by CellSearch, an EpCAM-dependent device, and ScreenCell®, a device size based. We found nontypical CTCs by CellSearch system and the immunofluorescence analysis performed on CTCs isolate by ScreenCell demonstrated the presence of single CTCs and CTC clusters. The molecular characterization of single CTCs and CTC clusters, using antibodies directed against EpCAM, CD34, cytokeratins (8, 18, and 19), and CD45, showed a great heterogeneity in CTC clusters. We believe that the present study may open a new scenario in the rare tumors: the introduction of the liquid biopsy and the molecular characterization of circulating tumor cells could lead to personalized targeted treatments and also for rare tumors.PMID: 29707475 PMCID: PMC5863319 DOI: 10.1155/2018/9718585
Nicolazzo C1, Colangelo L2, Corsi A3, Carpino G4, Gradilone A1, Sonato C2, Raimondi C1, Gaudio E5, Gazzaniga P1, Gianni W2.
Author information
1Department of Molecular Medicine, Circulating Tumor Cells Unit, Sapienza University of Rome, Viale Regina Elena 324, 00161 Rome, Italy.2Policlinico Umerto I, II Division of Internal Medicine and Geriatrics, Sapienza University of Rome, Viale del Policlinico 155, 00161 Rome, Italy.3Department of Radiological, Oncological and Anatomopathological Sciences, Sapienza University of Rome, Viale Regina Elena 324, 00161 Rome, Italy.4Department of Movement, Human and Health Sciences, Division of Health Sciences, Foro Italico University of Rome, Piazza Lauro De Bosis 6, 00135 Rome, Italy.5Department of Anatomical, Histological, Forensic Medicine and Orthopedics Sciences, Sapienza University of Rome, Via Alfonso Borelli 50, 00161 Rome, Italy.
2018February, 8th
Isolation and characterization of circulating melanoma cells by size filtration and fluorescent in-situ hybridization
Isolation and characterization of circulating melanoma cells by size filtrationand fluorescent in-situ hybridization.
Abstract
Isolation of circulating tumor cells (CTCs) from blood of melanoma patients has been difficult owing to inconsistent expression of surface antigens. Here we report on the isolation, detection, and characterization of CTCs from blood of melanoma patients using microfiltration and fluorescent in-situ hybridization (FISH). Two tubes of blood from 15 patients with advanced melanoma were collected. These two tubes subsequently underwent filtration through a membrane with pore sizes of 7.5 μm. Isolated cells from one tube were analyzed by FISH for RREB1 (6p24), MYB (6q32), SE6 (D6Z1), and CCND1 (11q13) and the other paired specimen was analyzed by immunofluorescence for HMB45, melanoma-associated antigen recognized by T cells-1, tyrosinase and melanogenesis associated transcription factor. We identified CTCs in 10 out of 13 melanoma samples by immunofluorescence (2.5-99 CTCs/3 ml of blood) and in 13 specimens by FISH (7.2-76 CTCs/3 ml of blood) with more CTCs identified by FISH in 10 out 13 samples. Two filters failed. Our results show that CTCs are detectable in the majority of patients with advanced melanoma. These tools will be useful in characterizing treatment related changes of melanoma in CTCs.
- PMID:
- 29406397
- DOI:
- 10.1097/CMR.0000000000000431
August, 23rd
Rapid and Sensitive Detection of Breast Cancer Cells in Patient Blood with Nuclease-Activated Probe Technology
Rapid and Sensitive Detection of Breast Cancer Cells in Patient Blood with Nuclease-Activated Probe Technology.
Abstract
A challenge for circulating tumor cell (CTC)-based diagnostics is the development of simple and inexpensive methods that reliably detect the diverse cells that make up CTCs. CTC-derived nucleases are one category of proteins that could be exploited to meet this challenge. Advantages of nucleases as CTC biomarkers include: (1) their elevated expression in many cancer cells, including cells implicated in metastasis that have undergone epithelial-to-mesenchymal transition; and (2) their enzymatic activity, which can be exploited for signal amplification in detection methods. Here, we describe a diagnostic assay based on quenched fluorescent nucleic acid probes that detect breast cancer CTCs via their nuclease activity. This assay exhibited robust performance in distinguishing breast cancer patients from healthy controls, and it is rapid, inexpensive, and easy to implement in most clinical labs. Given its broad applicability, this technology has the potential to have a substantive impact on the diagnosis and treatment of many cancers.
KEYWORDS:
breast cancer; cancer; circulating tumor cells; diagnostic markers; diagnostic nucleic acids; liquid biopsy; nucleases
- PMID:
- 28918054
- PMCID:
- PMC5577414
- DOI:
- 10.1016/j.omtn.2017.08.004
July, 23rd
Circulating tumor cells and microemboli can differentiate malignant and benign pulmonary lesions
Circulating tumor cells and microemboli can differentiate malignant and benign pulmonary lesions.
Abstract
The presence of circulating tumor cells (CTC) or microemboli (CTM) in the peripheral blood can theoretically anticipate malignancy of solid lesions in a variety of organs. We aimed to preliminarily assess this capability in patients with pulmonary lesions of suspected malignant nature. We used a cell-size filtration method (ScreenCell) and cytomorphometric criteria to detect CTC/CTM in a 3 mL sample of peripheral blood that was taken just before diagnostic percutaneous CT-guided fine needle aspiration (FNA) or biopsy of the suspicious lung lesion. At least one CTC/CTM was found in 47 of 67 (70%) patients with final diagnoses of lung malignancy and in none of 8 patients with benign pulmonary nodules. In particular they were detected in 38 (69%) of 55 primary lung cancers and in 9 (75%) of 12 lung metastases from extra-pulmonary cancers. Sensitivity of CTC/CTM presence for malignancy was 70.1% (95%CI: 56.9-83.1%), specificity 100%, positive predictive value 100% and negative predictive value 28.6% (95%CI: 11.9-45.3%). Remarkably, the presence of CTC/CTM anticipated the diagnosis of primary lung cancer in 3 of 5 patients with non-diagnostic or inconclusive results of FNA or biopsy, whereas CTC/CTM were not observed in 1 patient with sarcoidosis and 1 with amarthocondroma. These results suggest that presently, due to the low sensitivity, the search of CTC/CTM cannot replace CT guided percutaneous FNA or biopsy in the diagnostic work-up of patients with suspicious malignant lung lesions. However, the high specificity may as yet indicate a role in cases with non-diagnostic or inconclusive FNA or biopsy results that warrants to be further investigated.
KEYWORDS:
CT-guided fine needle aspiration.; circulating tumor cells; lung cancer; lung metastases; lung nodule
- PMID:
- 28819424
- PMCID:
- PMC5560139
- DOI:
- 10.7150/jca.1841
June, 23rd
Perioperative detection of circulating tumour cells in patients with lung cancer
Perioperative detection of circulating tumour cells in patients with lung cancer.
Abstract
Lung cancer is a leading cause of mortality and despite surgical resection a proportion of patients may develop metastatic spread. The detection of circulating tumour cells (CTCs) may allow for improved prediction of metastatic spread and survival. The current study evaluates the efficacy of the ScreenCell® filtration device, to capture, isolate and propagate CTCs in patients with primary lung cancer. Prior to assessment of CTCs, the present study detected cancer cells in a proof-of-principle- experiment using A549 human lung carcinoma cells as a model. Ten patients (five males and five females) with pathologically diagnosed primary non-small cell lung cancer undergoing surgical resection, had their blood tested for CTCs. Samples were taken from a peripheral vessel at the baseline, from the pulmonary vein draining the lobe containing the tumour immediately prior to division, a further central sample was taken following completion of the resection, and a final peripheral sample was taken three days post-resection. A significant increase in CTCs was observed from baseline levels following lung manipulation. No association was able to be made between increased levels of circulating tumour cells and survival or the development of metastatic deposits. Manipulation of the lung during surgical resection for non-small cell lung carcinoma results in a temporarily increased level of CTCs; however, no clinical impact for this increase was observed. Overall, the study suggests the ScreenCell® device has the potential to be used as a CTC isolation tool, following further work, adaptations and improvements to the technology and validation of results.
KEYWORDS:
circulating tumour cells; diagnosis; lung cancer
- PMID:
- 28789342
- PMCID:
- PMC5529936
- DOI:
- 10.3892/ol.2017.6366
Perioperative detection of circulating tumour cells in patients with lung cancer
June, 23rd
EpCAM-expressing circulating tumor cells in colorectal cancer
EpCAM-expressing circulating tumor cells in colorectal cancer.
Abstract
BACKGROUND:
Several studies have raised the issue of the inadequacy of CellSearch® to detect the entire pool of circulating tumor cells (CTCs) from blood of cancer patients, suggesting that cells expressing low levels of epithelial cell adhesion molecule (EpCAM) are not recognized by the capture reagent. In this exploratory study, we aimed to evaluate the status of EpCAM in CTCs isolated from a group of metastatic colorectal cancer patients, in 40% of whom, CTC had been found to be undetected by the CellSearch® system.
METHODS:
CTCs were analyzed using both a microfiltration method (ScreenCell) and CellSearch® in parallel. Furthermore, since EpCAM exists in 2 different variants, we investigated the presence of both its intracellular domain (EpICD) and extracellular domain (EpEX) through immunofluorescence staining of CTCs on filters.
RESULTS:
Results from immunofluorescence experiments demonstrated that, overall, EpICD and/or EpEX was expressed in 176 CTCs detected by ScreenCell, while the CellSearch® system was able to capture only 10 CTCs.
CONCLUSIONS:
This is the first demonstration that the low sensitivity of CellSearch® to detect CTCs in colorectal cancer patients is not due to the lack of EpCAM.
- PMID:
- 28604994
- DOI:
- 10.5301/ijbm.5000284
EpCAM-expressing circulating tumor cells in colorectal cancer
May, 23rd
Circulating Tumor DNA Reflects Tumor Metabolism Rather Than Tumor Burden in Chemotherapy-Naive Patients with Advanced Non-Small Cell Lung Cancer: 18F-FDG PET/CT Study
Circulating Tumor DNA Reflects Tumor Metabolism Rather Than Tumor Burden in Chemotherapy-Naive Patients with Advanced Non-Small Cell Lung Cancer: 18F-FDG PET/CT Study.
Abstract
We aimed to evaluate the relationships between circulating tumor cells (CTCs) or plasma cell-free DNA (cfDNA) on one side and a comprehensive range of 18F-FDG PET/CT-derived parameters on the other side in chemotherapy-naive patients with advanced non-small cell lung cancer (NSCLC). Methods: From a group of 79 patients included in a trial evaluating the role of pretreatment circulating tumor markers as predictors of prognosis in chemotherapy-naive patients with advanced NSCLC, we recruited all those who underwent 18F-FDG PET/CT for clinical reasons at our institution before inclusion in the trial (and thus just before chemotherapy). For each patient, a peripheral blood sample was collected at baseline for the evaluation of CTCs and cfDNA. CTCs were isolated by size using a filtration-based device and then morphologically identified and enumerated; cfDNA was isolated from plasma and quantified by a quantitative polymerase chain reaction using human telomerase reverse transcriptase. The following 18F-FDG PET/CT-derived parameters were computed: maximum diameter of the primary lesion (T), of the largest lymph node (N), and of the largest metastatic lesion (M); SUVmax; SUVmean; size-incorporated SUVmax; metabolic tumor volume; and total lesion glycolysis. All parameters were independently measured for T, N, and M. The associations among CTCs, cfDNA, and 18F-FDG PET/CT-derived parameters were evaluated by multivariate-analysis. Patients were divided into 2 groups according to the presence of either limited metastatic involvement (M1a or M1b due to extrathoracic lymph nodes only) or disseminated metastatic disease. The presence or absence of metabolically active bone lesions was also recorded for each patient, and patient subgroups were compared. Results: Thirty-seven patients recruited in the trial matched our PET-based criteria (24 men; age, 64.5 ± 8.1 y). SUVmax for the largest metastatic lesion was the only variable independently associated with baseline cfDNA levels (P = 0.016). Higher levels of cfDNA were detected in the subgroup of patients with metabolically active bone lesions (P = 0.02), but no difference was highlighted when patients with more limited metastatic disease were compared with patients with disseminated metastatic disease. Conclusion: The correlation of cfDNA levels with tumor metabolism, but not with metabolic tumor volume at regional or distant levels, suggests that cfDNA may better reflect tumor biologic behavior or aggressiveness rather than tumor burden in metastatic NSCLC.
TRIAL REGISTRATION:
ClinicalTrials.gov NCT02055144.
- PMID:
- 28450567
- DOI:
- 10.2967/jnumed.117.193201
Publications
2019October, 4th
Expression Profiling of Circulating Tumor Cells in Pancreatic Ductal Adenocarcinoma Patients: Biomarkers Predicting Overall Survival
Front Oncol. 2019 Sep 10;9:874. doi: 10.3389/fonc.2019.00874. eCollection 2019.
Amantini C1, Morelli MB1,2, Nabissi M2, Piva F3, Marinelli O1,2, Maggi F4, Bianchi F5, Bittoni A5, Berardi R5, Giampieri R5, Santoni G2.
Abstract
The interest in liquid biopsy is growing because it could represent a non-invasive prognostic or predictive tool for clinical outcome in patients with pancreatic ductal adenocarcinoma (PDAC), an aggressive and lethal disease. In this pilot study, circulating tumor cells (CTCs), CD16 positive atypical CTCs, and CTC clusters were captured and characterized in the blood of patients with PDAC before and after palliative first line chemotherapy by ScreenCell device, immunohistochemistry, and confocal microscopy analysis. Gene profiles were performed by digital droplet PCR in isolated CTCs, five primary PDAC tissues, and three different batches of RNA from normal human pancreatic tissue. Welsh’s t-test, Kaplan-Meier survival, and Univariate Cox regression analyses have been performed. Statistical analysis revealed that the presence of high CTC number in blood is a prognostic factor for poor overall survival and progression free survival in advanced PDAC patients, before and after first line chemotherapy. Furthermore, untreated PDAC patients with CTCs, characterized by high ALCAM, POU5F1B, and SMO mRNAs expression, have shorter progression free survival and overall survival compared with patients expressing the same biomarkers at low levels. Finally, high SHH mRNA levels are negatively associated to progression free survival, whereas high vimentin mRNA levels are correlated with the most favorable prognosis. By hierarchical clustering and correlation index analysis, two cluster gene signatures were identified in CTCs: the first, with high expression of VEGFA, NOTCH1, EPCAM, IHH, is the signature of PDAC patients before chemotherapy, whereas the second, with an enrichment in the expression of CD44, ALCAM, and POU5F1B stemness and pluripotency genes, is reported after palliative chemotherapy. Overall our data support the clinic value of the identification of CTC’s specific biomarkers to improve the prognosis and the therapy in advanced PDAC patients.
KEYWORDS:
atypical CTC; circulating tumor cells; digital droplet PCR; gene signature; overall survival; pancreatic cancerPMID: 31552188 PMCID: PMC6746928 DOI: 10.3389/fonc.2019.00874
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6746928/pdf/fonc-09-00874.pdf
Author information
1School of Biosciences and Veterinary Medicine, University of Camerino, Camerino, Italy.2School of Pharmacy, Experimental Medicine Section, University of Camerino, Camerino, Italy.3Department of Specialistic Clinical and Odontostomatological Sciences, Polytechnic University of Marche, Ancona, Italy.4Department of Molecular Medicine, Sapienza University of Rome, Rome, Italy.5Oncology Clinic, AOU Ospedali Riuniti, Polytechnic University of Marche, Ancona, Italy.
August, 5th
Circulating Tumor Cells in Right- and Left-Sided Colorectal Cancer
Nicolazzo C1, Raimondi C2, Gradilone A1, Emiliani A2, Zeuner A3, Francescangeli F3, Belardinilli F1, Seminara P2, Loreni F1, Magri V4, Tomao S2, Gazzaniga P5.
1 Department of Molecular Medicine, Circulating tumor cells Unit, Sapienza University of Rome, 00161 Rome, Italy.
2 Department of Radiological, Oncological and Pathological Sciences, Division of Medical Oncology, Sapienza University of Rome, 00161 Rome, Italy.
3 Department of Hematology, Oncology and Molecular Medicine, Istituto Superiore di Sanità, 00161 Rome, Italy.
4 Department of Surgical Sciences, Sapienza University of Rome, 00161 Rome, Italy.
5 Department of Molecular Medicine, Circulating tumor cells Unit, Sapienza University of Rome, 00161 Rome, Italy. paola.gazzaniga@uniroma1.it.
Abstract
Molecular alterations are not randomly distributed in colorectal cancer (CRC), but rather clustered on the basis of primary tumor location underlying the importance of colorectal cancer sidedness. We aimed to investigate whether circulating tumor cells (CTC) characterization might help clarify how different the patterns of dissemination might be relative to the behavior of left- (LCC) compared to right-sided (RCC) cancers. We retrospectively analyzed patients with metastatic CRC who had undergone standard baseline CTC evaluation before starting any first-line systemic treatment. Enumeration of CTC in left- and right-sided tumors were compared. The highest prognostic impact was exerted by CTC in left-sided primary cancer patients, even though the lowest median number of cells was detected in this subgroup of patients. CTC exhibit phenotypic heterogeneity, with a predominant mesenchymal phenotype found in CTC from distal compared to proximal primary tumors. Most CTC in RCC patients exhibited an apoptotic pattern. CTC in left-sided colon cancer patients exhibit a predominant mesenchymal phenotype. This might imply a substantial difference in the biology of proximal and distal cancers, associated with different patterns of tumor cells dissemination. The poor prognosis of right-sided CRC is not determined by the hematogenous dissemination of tumor cells, which appears to be predominantly a passive shedding of non-viable cells. Conversely, the subgroup of poor-prognosis left-sided CRC is reliably identified by the presence of mesenchymal CTC.
KEYWORDS:
CellSearch®; ScreenCell®; circulating tumor cells; colorectal cancer; epithelial-mesenchymal transition; prognosis; sidednessPMID: 31344798 DOI: 10.3390/cancers11081042
March, 21st
Non-Metastatic Esophageal Adenocarcinoma: Circulating Tumor Cells in the Course of Multimodal Tumor Treatment
February, 22nd
Liquid Biopsy in Rare Cancers: Lessons from Hemangiopericytoma
Abstract
Hemangiopericytoma (HPT) is a rare mesenchymal tumor of fibroblastic type and for its rarity is poorly studied. The most common sites of metastatic disease in patients with intracranial HPT are the bone, liver, and lung, suggestive for an hematogenous dissemination; for this reason, we investigated, for the first time, the presence of circulating tumor cells (CTCs) in hemangiopericytoma patient by CellSearch® and SceenCell® devices. Peripheral blood samples were drawn and processed by CellSearch, an EpCAM-dependent device, and ScreenCell®, a device size based. We found nontypical CTCs by CellSearch system and the immunofluorescence analysis performed on CTCs isolate by ScreenCell demonstrated the presence of single CTCs and CTC clusters. The molecular characterization of single CTCs and CTC clusters, using antibodies directed against EpCAM, CD34, cytokeratins (8, 18, and 19), and CD45, showed a great heterogeneity in CTC clusters. We believe that the present study may open a new scenario in the rare tumors: the introduction of the liquid biopsy and the molecular characterization of circulating tumor cells could lead to personalized targeted treatments and also for rare tumors.PMID: 29707475 PMCID: PMC5863319 DOI: 10.1155/2018/9718585
Nicolazzo C1, Colangelo L2, Corsi A3, Carpino G4, Gradilone A1, Sonato C2, Raimondi C1, Gaudio E5, Gazzaniga P1, Gianni W2.
Author information
1Department of Molecular Medicine, Circulating Tumor Cells Unit, Sapienza University of Rome, Viale Regina Elena 324, 00161 Rome, Italy.2Policlinico Umerto I, II Division of Internal Medicine and Geriatrics, Sapienza University of Rome, Viale del Policlinico 155, 00161 Rome, Italy.3Department of Radiological, Oncological and Anatomopathological Sciences, Sapienza University of Rome, Viale Regina Elena 324, 00161 Rome, Italy.4Department of Movement, Human and Health Sciences, Division of Health Sciences, Foro Italico University of Rome, Piazza Lauro De Bosis 6, 00135 Rome, Italy.5Department of Anatomical, Histological, Forensic Medicine and Orthopedics Sciences, Sapienza University of Rome, Via Alfonso Borelli 50, 00161 Rome, Italy.
2018February, 8th
Isolation and characterization of circulating melanoma cells by size filtration and fluorescent in-situ hybridization
Isolation and characterization of circulating melanoma cells by size filtrationand fluorescent in-situ hybridization.
Abstract
Isolation of circulating tumor cells (CTCs) from blood of melanoma patients has been difficult owing to inconsistent expression of surface antigens. Here we report on the isolation, detection, and characterization of CTCs from blood of melanoma patients using microfiltration and fluorescent in-situ hybridization (FISH). Two tubes of blood from 15 patients with advanced melanoma were collected. These two tubes subsequently underwent filtration through a membrane with pore sizes of 7.5 μm. Isolated cells from one tube were analyzed by FISH for RREB1 (6p24), MYB (6q32), SE6 (D6Z1), and CCND1 (11q13) and the other paired specimen was analyzed by immunofluorescence for HMB45, melanoma-associated antigen recognized by T cells-1, tyrosinase and melanogenesis associated transcription factor. We identified CTCs in 10 out of 13 melanoma samples by immunofluorescence (2.5-99 CTCs/3 ml of blood) and in 13 specimens by FISH (7.2-76 CTCs/3 ml of blood) with more CTCs identified by FISH in 10 out 13 samples. Two filters failed. Our results show that CTCs are detectable in the majority of patients with advanced melanoma. These tools will be useful in characterizing treatment related changes of melanoma in CTCs.
- PMID:
- 29406397
- DOI:
- 10.1097/CMR.0000000000000431
August, 23rd
Rapid and Sensitive Detection of Breast Cancer Cells in Patient Blood with Nuclease-Activated Probe Technology
Rapid and Sensitive Detection of Breast Cancer Cells in Patient Blood with Nuclease-Activated Probe Technology.
Abstract
A challenge for circulating tumor cell (CTC)-based diagnostics is the development of simple and inexpensive methods that reliably detect the diverse cells that make up CTCs. CTC-derived nucleases are one category of proteins that could be exploited to meet this challenge. Advantages of nucleases as CTC biomarkers include: (1) their elevated expression in many cancer cells, including cells implicated in metastasis that have undergone epithelial-to-mesenchymal transition; and (2) their enzymatic activity, which can be exploited for signal amplification in detection methods. Here, we describe a diagnostic assay based on quenched fluorescent nucleic acid probes that detect breast cancer CTCs via their nuclease activity. This assay exhibited robust performance in distinguishing breast cancer patients from healthy controls, and it is rapid, inexpensive, and easy to implement in most clinical labs. Given its broad applicability, this technology has the potential to have a substantive impact on the diagnosis and treatment of many cancers.
KEYWORDS:
breast cancer; cancer; circulating tumor cells; diagnostic markers; diagnostic nucleic acids; liquid biopsy; nucleases
- PMID:
- 28918054
- PMCID:
- PMC5577414
- DOI:
- 10.1016/j.omtn.2017.08.004
July, 23rd
Circulating tumor cells and microemboli can differentiate malignant and benign pulmonary lesions
Circulating tumor cells and microemboli can differentiate malignant and benign pulmonary lesions.
Abstract
The presence of circulating tumor cells (CTC) or microemboli (CTM) in the peripheral blood can theoretically anticipate malignancy of solid lesions in a variety of organs. We aimed to preliminarily assess this capability in patients with pulmonary lesions of suspected malignant nature. We used a cell-size filtration method (ScreenCell) and cytomorphometric criteria to detect CTC/CTM in a 3 mL sample of peripheral blood that was taken just before diagnostic percutaneous CT-guided fine needle aspiration (FNA) or biopsy of the suspicious lung lesion. At least one CTC/CTM was found in 47 of 67 (70%) patients with final diagnoses of lung malignancy and in none of 8 patients with benign pulmonary nodules. In particular they were detected in 38 (69%) of 55 primary lung cancers and in 9 (75%) of 12 lung metastases from extra-pulmonary cancers. Sensitivity of CTC/CTM presence for malignancy was 70.1% (95%CI: 56.9-83.1%), specificity 100%, positive predictive value 100% and negative predictive value 28.6% (95%CI: 11.9-45.3%). Remarkably, the presence of CTC/CTM anticipated the diagnosis of primary lung cancer in 3 of 5 patients with non-diagnostic or inconclusive results of FNA or biopsy, whereas CTC/CTM were not observed in 1 patient with sarcoidosis and 1 with amarthocondroma. These results suggest that presently, due to the low sensitivity, the search of CTC/CTM cannot replace CT guided percutaneous FNA or biopsy in the diagnostic work-up of patients with suspicious malignant lung lesions. However, the high specificity may as yet indicate a role in cases with non-diagnostic or inconclusive FNA or biopsy results that warrants to be further investigated.
KEYWORDS:
CT-guided fine needle aspiration.; circulating tumor cells; lung cancer; lung metastases; lung nodule
- PMID:
- 28819424
- PMCID:
- PMC5560139
- DOI:
- 10.7150/jca.1841
June, 23rd
Perioperative detection of circulating tumour cells in patients with lung cancer
Perioperative detection of circulating tumour cells in patients with lung cancer.
Abstract
Lung cancer is a leading cause of mortality and despite surgical resection a proportion of patients may develop metastatic spread. The detection of circulating tumour cells (CTCs) may allow for improved prediction of metastatic spread and survival. The current study evaluates the efficacy of the ScreenCell® filtration device, to capture, isolate and propagate CTCs in patients with primary lung cancer. Prior to assessment of CTCs, the present study detected cancer cells in a proof-of-principle- experiment using A549 human lung carcinoma cells as a model. Ten patients (five males and five females) with pathologically diagnosed primary non-small cell lung cancer undergoing surgical resection, had their blood tested for CTCs. Samples were taken from a peripheral vessel at the baseline, from the pulmonary vein draining the lobe containing the tumour immediately prior to division, a further central sample was taken following completion of the resection, and a final peripheral sample was taken three days post-resection. A significant increase in CTCs was observed from baseline levels following lung manipulation. No association was able to be made between increased levels of circulating tumour cells and survival or the development of metastatic deposits. Manipulation of the lung during surgical resection for non-small cell lung carcinoma results in a temporarily increased level of CTCs; however, no clinical impact for this increase was observed. Overall, the study suggests the ScreenCell® device has the potential to be used as a CTC isolation tool, following further work, adaptations and improvements to the technology and validation of results.
KEYWORDS:
circulating tumour cells; diagnosis; lung cancer
- PMID:
- 28789342
- PMCID:
- PMC5529936
- DOI:
- 10.3892/ol.2017.6366
Perioperative detection of circulating tumour cells in patients with lung cancer
June, 23rd
EpCAM-expressing circulating tumor cells in colorectal cancer
EpCAM-expressing circulating tumor cells in colorectal cancer.
Abstract
BACKGROUND:
Several studies have raised the issue of the inadequacy of CellSearch® to detect the entire pool of circulating tumor cells (CTCs) from blood of cancer patients, suggesting that cells expressing low levels of epithelial cell adhesion molecule (EpCAM) are not recognized by the capture reagent. In this exploratory study, we aimed to evaluate the status of EpCAM in CTCs isolated from a group of metastatic colorectal cancer patients, in 40% of whom, CTC had been found to be undetected by the CellSearch® system.
METHODS:
CTCs were analyzed using both a microfiltration method (ScreenCell) and CellSearch® in parallel. Furthermore, since EpCAM exists in 2 different variants, we investigated the presence of both its intracellular domain (EpICD) and extracellular domain (EpEX) through immunofluorescence staining of CTCs on filters.
RESULTS:
Results from immunofluorescence experiments demonstrated that, overall, EpICD and/or EpEX was expressed in 176 CTCs detected by ScreenCell, while the CellSearch® system was able to capture only 10 CTCs.
CONCLUSIONS:
This is the first demonstration that the low sensitivity of CellSearch® to detect CTCs in colorectal cancer patients is not due to the lack of EpCAM.
- PMID:
- 28604994
- DOI:
- 10.5301/ijbm.5000284
EpCAM-expressing circulating tumor cells in colorectal cancer
May, 23rd
Circulating Tumor DNA Reflects Tumor Metabolism Rather Than Tumor Burden in Chemotherapy-Naive Patients with Advanced Non-Small Cell Lung Cancer: 18F-FDG PET/CT Study
Circulating Tumor DNA Reflects Tumor Metabolism Rather Than Tumor Burden in Chemotherapy-Naive Patients with Advanced Non-Small Cell Lung Cancer: 18F-FDG PET/CT Study.
Abstract
We aimed to evaluate the relationships between circulating tumor cells (CTCs) or plasma cell-free DNA (cfDNA) on one side and a comprehensive range of 18F-FDG PET/CT-derived parameters on the other side in chemotherapy-naive patients with advanced non-small cell lung cancer (NSCLC). Methods: From a group of 79 patients included in a trial evaluating the role of pretreatment circulating tumor markers as predictors of prognosis in chemotherapy-naive patients with advanced NSCLC, we recruited all those who underwent 18F-FDG PET/CT for clinical reasons at our institution before inclusion in the trial (and thus just before chemotherapy). For each patient, a peripheral blood sample was collected at baseline for the evaluation of CTCs and cfDNA. CTCs were isolated by size using a filtration-based device and then morphologically identified and enumerated; cfDNA was isolated from plasma and quantified by a quantitative polymerase chain reaction using human telomerase reverse transcriptase. The following 18F-FDG PET/CT-derived parameters were computed: maximum diameter of the primary lesion (T), of the largest lymph node (N), and of the largest metastatic lesion (M); SUVmax; SUVmean; size-incorporated SUVmax; metabolic tumor volume; and total lesion glycolysis. All parameters were independently measured for T, N, and M. The associations among CTCs, cfDNA, and 18F-FDG PET/CT-derived parameters were evaluated by multivariate-analysis. Patients were divided into 2 groups according to the presence of either limited metastatic involvement (M1a or M1b due to extrathoracic lymph nodes only) or disseminated metastatic disease. The presence or absence of metabolically active bone lesions was also recorded for each patient, and patient subgroups were compared. Results: Thirty-seven patients recruited in the trial matched our PET-based criteria (24 men; age, 64.5 ± 8.1 y). SUVmax for the largest metastatic lesion was the only variable independently associated with baseline cfDNA levels (P = 0.016). Higher levels of cfDNA were detected in the subgroup of patients with metabolically active bone lesions (P = 0.02), but no difference was highlighted when patients with more limited metastatic disease were compared with patients with disseminated metastatic disease. Conclusion: The correlation of cfDNA levels with tumor metabolism, but not with metabolic tumor volume at regional or distant levels, suggests that cfDNA may better reflect tumor biologic behavior or aggressiveness rather than tumor burden in metastatic NSCLC.
TRIAL REGISTRATION:
ClinicalTrials.gov NCT02055144.
- PMID:
- 28450567
- DOI:
- 10.2967/jnumed.117.193201
To combine circulating tumor cell (CTC) isolation by filtration and immunohistochemistry to investigate the presence of CTCs in low, intermediate, and high-risk prostate cancer (PCa). CTCs isolated from these risk groups stained positive for both cytokeratin and androgen receptors, but negative for CD45.
PATIENTS AND METHODS:
Blood samples from 41 biopsy confirmed patients with PCa at different clinical stages such as low, intermediate, and high risk were analyzed. The samples were processed with the ScreenCell filtration device and PCa CTCs were captured for all patients. The isolated CTCs were confirmed PCa CTCs by the presence of androgen receptors and cytokeratins 8, 18, and 19 that occurred in the absence of CD45 positivity. PCa CTC nuclear sizes were measured using the TeloView program.
RESULTS:
The filtration-based isolation method used permitted the measurement of the average nuclear size of the captured CTCs. CTCs were identified by immunohistochemistry in low, intermediate, and high-risk groups of patients with PCa.
CONCLUSION:
CTCs may be found in all stages of PCa. These CTCs can be used to determine the level of genomic instability at any stage of PCa; this will, in the future, enable personalized patient management.
January, 6th
Detection of Circulating Tumour Cells and Survival of Patients with Non-small Cell Lung Cancer.
Detection of Circulating Tumour Cells and Survival of Patients with Non-small Cell Lung Cancer.
- 1Department of Thoracic Surgery, Royal Brompton and Harefield NHS Foundation Trust, London, U.K.
- 2Department of Histopathology, Royal Brompton and Harefield NHS Foundation Trust, London, U.K.
- 3Department of Thoracic Surgery, Royal Brompton and Harefield NHS Foundation Trust, London, U.K. v.anikin@rbht.nhs.uk.
Abstract
BACKGROUND:
Detection of circulating tumour cells (CTCs) in the peripheral blood of lung cancer patients may predict survival. Various platforms exist that allow capture of these cells for further analysis; little work however, has been done with the ScreenCell device, an antibody-independent CTC platform. The aim of our study was to evaluate the ScreenCell device for detection of CTCs in lung cancer patients and to establish correlations of these findings with survival.
MATERIALS AND METHODS:
Twenty-three patients, nine males, and fourteen females, underwent surgical treatment from February to May 2014 for non-small cell lung cancer. Thirteen patients had adenocarcinoma and ten squamous cell carcinoma, while eight were at an early stage (I-II) and five at a later stage (III-IV). Blood samples were obtained prior to surgery and following filtration through the ScreenCell device, were independently reviewed by 2 consultant pathologists.
RESULTS:
The pathologists were able to independently identify CTCs in 78.3% (N=18) and 73.9% (N=17) of the cases examined, with overall 80.6% in early stages compared to 60.0% in late stages. The median survival times of positive vs. negative for CTC patients were 1011 and 711 days respectively, with a survival percentage rate of 77.8% and 60% in positive and negative CTC cohorts respectively.
CONCLUSION:
The results of this study suggest that the presence of CTCs analyzed by ScreenCell did not necessarily lead to a poorer prognosis in patients with lung cancer after curative surgery.
Detection of Circulating Tumour Cells and Survival of Patients with Non-small Cell Lung Cancer
October, 3rd
Detection and Characterization of Circulating Tumor Associated Cells in Metastatic Breast Cancer
Article
Detection and Characterization of Circulating Tumor Associated Cells in Metastatic Breast Cancer
Zhaomei Mu 1,*, Naoual Benali-Furet 2, Georges Uzan 2, Anaëlle Znaty 2, Zhong Ye 3,
Carmela Paolillo 4, Chun Wang 3, Laura Austin 3, Giovanna Rossi 1, Paolo Fortina 4,5,
Hushan Yang 3 and Massimo Cristofanilli 1,*
1 Department of Medicine-Hematology and Oncology, Robert H Lurie Comprehensive Cancer Center,
Feinberg School of Medicine, Northwestern University, Chicago, IL 60611, USA; giovirossi85@yahoo.it
2 ScreenCell SA, Sarcelles 95200, France; benali@screencell.com (N.B.-F.); guzan@screencell.com (G.U.);
aznaty@screencell.com (A.Z.)
3 Department of Medical Oncology, Sidney Kimmel Cancer Center, Thomas Jefferson University,
Philadelphia, PA 19107, USA; Zhong.Ye@jefferson.edu (Z.Y.); Chun.Wang@jefferson.edu (C.W.);
laustin@gmail.com (L.A.); hushan.yang@jefferson.edu (H.Y.)
4 Department of Cancer Biology, Sidney Kimmel Cancer Center, Thomas Jefferson University,
Philadelphia, PA 19107, USA; carmela.Paolillo@jefferson.edu (C.P.); paolo.Fortina@jefferson.edu (P.F.)
5 Department of Molecular Medicine, University of Rome “Sapienza”, Rome 00185, Italy
* Correspondence: zhaomei.mu@northwestern.edu (Z.M.); massimo.cristofanilli@nm.org (M.C.);
Tel.: +1-312-503-5489 (Z.M.); +1-312-503-5488 (M.C.)
Academic Editor: Dario Marchetti
Received: 5 August 2016; Accepted: 23 September 2016; Published: 30 September 2016
Abstract: The availability of blood-based diagnostic testing using a non-invasive technique holds
promise for real-time monitoring of disease progression and treatment selection. Circulating tumor
cells (CTCs) have been used as a prognostic biomarker for the metastatic breast cancer (MBC).
The molecular characterization of CTCs is fundamental to the phenotypic identification of malignant
cells and description of the relevant genetic alterations that may change according to disease
progression and therapy resistance. However, the molecular characterization of CTCs remains
a challenge because of the rarity and heterogeneity of CTCs and technological difficulties in the
enrichment, isolation and molecular characterization of CTCs. In this pilot study, we evaluated
circulating tumor associated cells in one blood draw by size exclusion technology and cytological
analysis. Among 30 prospectively enrolled MBC patients, CTCs, circulating tumor cell clusters (CTC
clusters), CTCs of epithelial–mesenchymal transition (EMT) and cancer associated macrophage-like
cells (CAMLs) were detected and analyzed. For molecular characterization of CTCs, size-exclusion
method for CTC enrichment was tested in combination with DEPArray™ technology, which allows
the recovery of single CTCs or pools of CTCs as a pure CTC sample for mutation analysis. Genomic
mutations of TP53 and ESR1 were analyzed by targeted sequencing on isolated 7 CTCs from a patient
with MBC. The results of genomic analysis showed heterozygous TP53 R248W mutation from one
single CTC and pools of three CTCs, and homozygous TP53 R248W mutation from one single CTC and
pools of two CTCs. Wild-type ESR1 was detected in the same isolated CTCs. The results of this study
reveal that size-exclusion method can be used to enrich and identify circulating tumor associated
cells, and enriched CTCs were characterized for genetic alterations in MBC patients, respectively.
Keywords: metastatic breast cancer (MBC); circulating tumor associated cells; circulating tumor
cells (CTCs); circulating tumor cell clusters (CTC clusters); epithelial–mesenchymal transition (EMT);
cancer associated macrophage-like cells (CAMLs); size-exclusion technology
May, 30th
Droplet Digital PCR of circulating tumor cells from colorectal cancer patients can predict KRAS mutations before surgery
Droplet Digital PCR of circulating tumor cells from colorectal cancer patients can predict KRAS mutations before surgery.
Jérôme Alexandre Denis1,2,3, Alexia Patroni4, Erell Guillerm1,5, Dominique Pépin2, Naoual Benali-Furet6, Janine Wechsler6, Gilles Manceau1,4, Maguy Bernard2, Florence Coulet1,5; Annette K. Larsen3, Mehdi Karoui1,4, Jean-Marc Lacorte1,2, 7
1. Sorbonne Universités, UPMC Univ. Paris 06, F-75005, Paris, France.
2. Assistance Publique-Hôpitaux de Paris, Pitié-Salpêtrière Hospital, Department of Oncology and Endocrine Biochemistry, Paris, France.
3. Cancer Biology and Therapeutics, Centre de Recherche Saint-Antoine, Institut National de la Santé et de la Recherche Médicale (INSERM) U938 and Institut Universitaire de Cancérologie (IUC), Université Pierre et Marie Curie (UPMC), Sorbonne Universities, Paris, France.
4. Assistance Publique-Hôpitaux de Paris, Pitié-Salpêtrière Hospital, Department of Digestive and Hepato-Pancreato-Biliary Surgery, Paris, France.
5. Assistance Publique-Hôpitaux de Paris, Pitié-Salpêtrière Hospital, Department of oncogenetics and molecular angiogenetics, Paris, France.
6. ScreenCell SA, Sarcelles, France
7. INSERM, UMR_S 1166, Institute of cardiometabolism and nutrition. ICAN. Paris, France
Abstract:
In colorectal cancer (CRC), KRAS mutations are a strong negative predictor for treatment with the EGFR-targeted antibodies cetuximab and panitumumab. Since it can be difficult to obtain appropriate tumor tissues for KRAS genotyping, alternative methods are required. Circulating tumor cells (CTCs) are believed to be representative of the tumor in real time. In this study, we explored the capacity of a size-based device for capturing CTCs coupled with a multiplex KRAS screening assay using droplet digital PCR (ddPCR). We showed that it is possible to detect a mutant ratio of 0.05 % and less than one KRAS mutant cell per mL total blood with ddPCR compared to about 0.5% and 50-75 cells for TaqMeltPCR and HRM. Next, CTCs were isolated from the blood of 35 patients with CRC at various stage of the disease. KRAS genotyping was successful for 86% (30/35) of samples with a KRAS codon 12/13 mutant ratio of 57% (17/30). In contrast, only one patient was identified as KRAS mutant when size-based isolation was combined with HRM or TaqMeltPCR. KRAS status was then determined for the 26 available formalin-fixed paraffin-embedded tumors using standard procedures. The concordance between the CTCs and the corresponding tumor tissues was 77% with a sensitivity of 83%. Taken together, the data presented here suggest that is feasible to detect KRAS mutations in CTCs from blood samples of CRC patients which are predictive for those found in the tumor. The minimal invasive nature of this procedure in combination with the high sensitivity of ddPCR might provide in the future an opportunity to monitor patients throughout the course of disease on multiple levels including early detection, prognosis, treatment and relapse as well as to obtain mechanistic insight with respect to tumor invasion and metastasis.
The paper has been accepted for publication in Molecular Oncology, in press.
2015December, 10th
SABCs 2015: Detection and Characterization of CTCs Isolated by ScreenCell® Size Exclusion Technology in Metastatic Breast Cancer
SABCs 2015 : Poster Session 2 – Thursday, December 10 7:30 am – 9:00 am
https://www.sabcs.org/Program/Poster-Sessions/Poster-Session-2
Background: Circulating Tumor cells (CTCs) detection has prognostic and predictive implications in patients with metastatic breast cancer (MBC). Genomic and phenotypic analysis of CTCs hold enormous promise as blood-based molecular characterization and monitoring disease progression and treatment benefit with a strong potential to be translated into more individualized targeted treatments. FDA-approved CellSearch™ detection allows only enumeration of CTCs expressing EpCAM without molecular characterization. CTCs represent very heterogeneous populations of tumorigenic cancer cells and some subpopulations have undergone epithelial-Mesenchymal transition (EMT), which is associated metastasis process and an unfavourable outcome. EpCAM-based enrichment technique has failed to detect EMT subpopulations due to the decreased expression or loss of epithelial markers. Non-EpCAM-based approaches are needed for identifying EMT CTCs. The ScreenCell® devices are single-use and low-cost innovative devices that use a filter for enrichment-free isolation of CTCs by a two-steps combining size-based separation and staining using different markers. The DEPArray™ system is the ideal downstream isolation system to collect single or pooled CTCs for molecular and genetic analysis. In this study, we evaluated the feasibility of achieving CTCs detection/enumeration using ScreenCell® filtration followed by single cell isolation with the DEPArray™ in MBC patients.
Methods: The first part of the study consisted in evaluating CTCs detection/enumeration in 30 patients with stage III and stage IV breast cancer. 3 mL of whole blood in an EDTA or Transfix tubes was collected and processed on the ScreenCell® Cyto device following the instructions of the supplier. CTCs were stained with cytokeratin (CK-8, 18, and 19), leukocyte antigen (CD45), and a nuclear dye (DAPI) and counted under fluorescence microscope. CTCs were identified as positive staining for CK and DAPI and negative staining for CD45 (CK+/DAPI+CD45-). In the second part, After enrichment, CTCs were stained with CK, CD45, and DAPI and sorted with DEPArray™ Platform (Silicon tems, Inc). Single CTCs were collected and the DNA of each single CTCs was amplified with Ampli1™ WGA kit, and the genome integrity index (GII) was assessed by Ampli1™ QC kit (Silicon tems, Inc). Library was constructed and whole exome sequencing (WES) of DNA mutations was conducted.
Results: Twenty patient samples had CTCs detected (66.7%), the number of CTCs was 1 to 347 per 3.0 ml of whole blood. CTC-clusters were detected in 7 patient samples (23.3%). Single CTCs were collected on DEPArray™ platform after enrichment with ScreenCell filtration. GII was confirmed with the presence of short, medium, and long DNA fragments (3 to 4 PCR bands) in the WGA library by PCR-based assay. All collected CTCs showed high GII as measured by Ampli1™ QC kit (GII ≥ 3) for WES of DNA mutations. The data analysis of WES results is under processing.
Conclusions: ScreenCell® filtration is simple and effective devices to isolate CTCs and identify CTC-clusters. Isolation of single cells for molecular analysis using the combination of ScreenCell® filtration and DEPArray™ Platform is feasible for genetic characterization of CTCs.
Authors: Zhaomei Mu1, Naoual Benali-Furet2, Georges Uzan2, Zhong Ye1, Carmela Paolillo1, Laura Austin1, Chun Wang1, Rebecca Jaslow1, Hushan Yang1, Paolo Fortina1,
Massimo Cristofanilli1
Institutions: 1Department of Medical Oncology, Sidney Kimmel Cancer Center, Thomas Jefferson University, Philadelphia, PA, United States, 19107 and 2ScreenCell, Sarcelles, France, 95200.
SABCs-Poster P2 02 14 Massimo Cristofanilli dec 2015
October, 9th
A comparative analysis of cancer hotspot mutation profiles in circulating tumour cells, circulating tumour DNA and matched primary lung tumour
A comparative analysis of cancer hotspot mutation profiles in circulating tumour cells, circulating tumour DNA and matched primary lung tumour
Maria Leung2, Maxim Freidin1, Dasha Freidina1, Sanjay Popat3, Andrew Nicholson2, Alexandra Rice2, Angeles Montero Fernandez2, Eric Lim1,2
1National Heart and Lung Institute, Imperial College London; 2Royal Brompton Hospital, London; 3Royal Marsden Hospital, London
The aim of our work is to report the concordances between CTCs and ctDNA versus the primary FFPE tumour mutations using a NGS hotspot panel.
“Our results suggest on a next generation sequencing platform that the global genetic variant profile between DNA extracted from CTC had good agreement with FFPE primary tumour tissue, and the agreement between ctDNA and FFPE was much poorer!” said consultant thoracic surgeon Dr Eric Lim from Royal Brompton Hospital, London.
July, 30th
Prevalence and number of circulating tumour cells and microemboli at diagnosis of advanced NSCLC.
Prevalence and number of circulating tumour cells and microemboli at diagnosis of advanced NSCLC.
Abstract
PURPOSE:
Timing and magnitude of blood release of circulating tumour cells (CTC) and circulating tumour microemboli (CTM) from primary solid cancers are uncertain. We investigated prevalence and number of CTC and CTM at diagnosis of advanced non-small cell lung cancer (NSCLC).
METHODS:
Twenty-eight consecutive patients with suspected stage III-IV lung cancer gave consent to provide 15 mL of peripheral blood soon before diagnostic CT-guided fine-needle aspiration biopsy (FNAB). CTC and CTM (clusters of ≥3 CTC) were isolated by cell size filtration (ScreenCell), identified and counted by cytopathologists using morphometric criteria and (in 6 cases) immunostained for vimentin.
RESULTS:
FNAB demonstrated NSCLC in 26 cases. At least one CTC/3 mL blood (mean 6.8 ± 3.7) was detected in 17 (65 %) and one CTM (mean 4.5 ± 3.3) in 15 (58 %) of 26 NSCLC cases. No correlation between number of CTC or CTM and tumour type or stage was observed. Neoplastic cells from both FNA and CTC/CTM were positive for vimentin but heterogeneously.
CONCLUSIONS:
CTC can be detected in two-thirds and CTM in more than half of patients with advanced NSCLC at diagnosis. Reasons underlying lack of CTC and CTM in some advanced lung cancers deserve further investigations.
July, 2nd
Circulating Tumor Cells in Diagnosing Lung Cancer: Clinical and Morphologic Analysis.
Circulating Tumor Cells in Diagnosing Lung Cancer: Clinical and Morphologic Analysis.
Fiorelli A, Accardo M, Carelli E, Angioletti D, Santini M, Di Domenico M.
Ann Thorac Surg. 2015 Jun;99(6):1899-905. doi: 10.1016/j.athoracsur.2014.11.049. Epub 2015 Feb 10.
Abstract
BACKGROUND:
The purpose of this study was to evaluate the value of circulating non-hematologic cells to differentiate benign from malignant lung lesions and their comparison with clinico-histologic features of corresponding primary lesions.
METHODS:
Circulating cells were isolated by size method from peripheral blood of 77 patients with malignant (n = 60) and benign (n = 17) lunglesions. They were morphologically classified as cells with malignant feature; cells with uncertain malignant feature; and cells with benign feature; then statistically correlated with clinico-cytopathologic characteristics of corresponding lung lesion.
RESULTS:
Malignant circulating cells were detected in 54 of 60 (90%) malignant patients, and in 1 of 17 (5%) benign patients; benign circulating cellsin 1 of 60 (1%) malignant patients and in 15 of 17 (88%) benign patients; and circulating cells with uncertain malignant aspect in 5 of 60 (8%) malignant patients and 1 of 17 (5%) benign patients. For a malignant circulating cells count greater than 25, sensitivity and specificity were 89% and 100%, respectively. The count was significantly correlated with stage, size, and standard uptake value of primary tumor. In 39 of 54 (72%) cases, the malignant circulating cells allowed a specific histologic diagnosis of the corresponding primary tumor after immunohistochemical analysis.
CONCLUSIONS:
Malignant circulating cells may be a valid marker in the diagnostic workup of lung lesions. However, our resuts should be corroborated by larger future studies especially for patients having small nodules.
Circulating Tumor Cells in Diagnosing Lung Cancer Clinical and Morphologic Analysis
July, 2nd
Circulating Tumour Cells in Patients with Malignant Lung Tumors Undergoing Radio-frequency Ablation
Circulating Tumour Cells in Patients with Malignant Lung TumorsUndergoing Radio-frequency Ablation.
Abstract
BACKGROUND/AIM:
Radiofrequency ablation (RFA) is an increasingly utilised technique in patients with surgically-untreatable lesions. The effect of this therapy on circulating tumor cells (CTCs) is unknown. As far as we are aware of, this is the first study to evaluate the effects of RFA on CTCs in patients with malignant lung tumors immediately post-treatment.
PATIENTS AND METHODS:
Nine patients with primary or metastatic lung tumors underwent RFA therapy from June to November 2013. Blood samples were taken before and after RFA, and filtered through the ScreenCell CTC capture device.
RESULTS:
A general increase in CTCs in 7 out of the 9 cases was found, the largest increases were seen in the metastatic group.
CONCLUSION:
This study demonstrates that the manipulation and ablative procedure of lung tumors leads to immediate dissemination of tumor cells, the effects of which are unknown and require further investigation.
July, 2nd
Circulating tumour cells in patients with lung cancer undergoing endobronchial cryotherapy.
Circulating tumour cells in patients with lung cancer undergoingendobronchial cryotherapy.
Abstract
Early diagnosis of lung cancer still poses a major issue, with a large proportion of patients diagnosed at late stages. Therapeutic options and treatment remain limited in these patients. In most cases only palliative therapies are available to alleviate any severe symptoms. Endobronchial cryotherapy (EC) is one form of palliative treatment offered to patients with obstructive airway tumours. Although successful, the impact on circulating tumour cell (CTCs) spread has not been investigated in detail. This study recruited 20 patients awaiting EC treatment. Baseline and post EC blood samples were analysed for presence of CTCs. Results showed an increase in CTCs following EC in 75% of patients. Significant increases were noticeable in some cases. Although EC is a well-accepted modality of treatment to alleviate symptoms, it may lead to an increase in CTCs, which in turn may have implications for tumour dissemination and metastatic spread.
Circulating tumour cells in patients with lung cancer undergoing endobronchial cryotherapy.
April, 2nd
Circulating Tumor Cells Found in Patients With Localized and Advanced Pancreatic Cancer.
Circulating Tumor Cells Found in Patients With Localized and Advanced Pancreatic Cancer.
Kulemann B1, Pitman MB, Liss AS, Valsangkar N, Fernández-Del Castillo C, Lillemoe KD, Hoeppner J, Mino-Kenudson M, Warshaw AL, Thayer SP.
Abstract
OBJECTIVES:
Isolation of circulating tumor cells (CTCs) holds the promise of diagnosing and molecular profiling cancers from a blood sample. Here, we test a simple new low-cost filtration device for CTC isolation in patients with pancreatic ductal adenocarcinoma (PDAC).
METHODS:
Peripheral blood samples drawn from healthy donors and PDAC patients were filtered using ScreenCell devices, designed to capture CTCs for cytologic and molecular analysis. Giemsa-stained specimens were evaluated by a pancreatic cytopathologist blinded to the histological diagnosis. Circulating tumor cell DNA was subjected to KRAS mutational analysis.
RESULTS:
Spiking experiments demonstrated a CTC capture efficiency as low as 2 cells/mL of blood. Circulating tumor cells were identified by either malignant cytology or presence of KRAS mutation in 73% of 11 patients (P = 0.001). Circulating tumor cells were identified in 3 of 4 patients with early (≤American Joint Committee on Cancer stage IIB) and in 5 of 7 patients with advanced (≥ American Joint Committee on Cancer stage III) PDAC. No CTCs were detected in blood from 9 health donors.
CONCLUSIONS:
Circulating tumor cells can be found in most patients with PDAC of any stage, whether localized, locally advanced, or metastatic. The ability to capture, cytologically identify, and genetically analyze CTCs suggests a possible tool for the diagnosis and characterization of genetic alterations of PDAC.
March, 6th
BREAKTHROUGH IN DIAGNOSIS OF PROSTATE CANCER
Dr. Sabine Mai of the Manitoba Institute of Cell Biology, CancerCare Manitoba and University of Manitoba, in collaboration with Drs. Drachenberg and Saranchuk at the Manitoba Prostate Centre, report on the use of Screencell technology for their researches on Prostate cancer.
BREAKTHROUGH IN DIAGNOSIS OF PROSTATE CANCER
WINNIPEG, MB: Dr. Sabine Mai of the Manitoba Institute of Cell Biology, CancerCare Manitoba and University of Manitoba, in collaboration with Drs. Drachenberg and Saranchuk at the Manitoba Prostate Centre, have made advances in examining circulating tumor cells (CTCs) from the blood of prostate cancer patients. The encouraging new advancements were made possible, in part, with funds raised by the Manitoba Motorcycle Ride for Dad.
Dr. Mai, Director of the Genomic Centre for Cancer Research and Diagnosis and head of the test project, presented findings about the new blood test at a recent meeting in Boston. She co-founded 3D Signatures Inc., for commercialization of the test. “The new blood test for prostate cancer will be less invasive with a potential to be more accurate,” said Dr. Mai, who is working to get certification for the test from Health Canada.
As reported by Frank Luba in the Vancouver Province (02/03/2015), Dr. Oliver Prange (Vancouver) said the new test is focused on the intermediate group of men diagnosed with prostate cancer, which comprises about 30 per cent of the total cases. Men in the intermediate group will either receive active surveillance or aggressive cancer therapy, depending on the diagnosis. “The team looks at the structural arrangement of the chromosome component which eliminates what is a bit of a guessing game,” said Dr. Prange. “Early evidence clearly clustered patients into risk groups. We hope to accurately predict which patients will stay indolent and which will progress,” he said.
“We hope that it will dramatically change the prognostic outlook of intermediate-risk prostate cancer patients,” said Dr. Mai.
Dr. Stuart Edmonds of Prostate Cancer Canada is encouraged by the new test. “I think it’s very promising,” Edmonds said from Toronto, where he is Prostate Cancer Canada’s vice-president of research, health promotions and survivorship. “We need to have a better test to distinguish between the aggressive disease that a man will die of rather than the more indolent, or non-aggressive disease, that a man will die with,” said Edmonds. Research is also under way to see if the new test could be used in the prognosis of other cancers, such as breast cancer, Hodgkin’s lymphoma and multiple myeloma.
Prostate cancer is the most commonly diagnosed cancer among men. It is estimated 23,600 men in Canada will be diagnosed with the cancer in 2015 and 4,000 would die from the disease.
Since 2009, over $860,000 has been raised by the Manitoba Motorcycle Ride for Dad for prostate cancer research and education. Dr. Mai’s research is proof-positive Manitobans are making a real difference. “Thank you to all Manitoba Motorcycle Ride for Dad volunteers, riders, donors and sponsors for your strong support of the project,” added Dr. Mai.
A video re: Dr. Mai’s project is found here: Dr. Sabine Mai Research Project Visit: www.ridefordad.ca/manitoba for more information about the Motorcycle Ride for Dad.
Contacts:
Dr. Sabine Mai: (204) 787-2135 sabine.mai@umanitoba.ca
Ed Johner, Spokesperson, MRFD: (204) 794-5602 edjohner@icloud.com
Moe Sabourin, Co-Chair, MRFD: (204) 228-4301 MSabourin@wpa.mb.ca
Kirk Van Alstyne, Co-Chair, MRFD: (204) 470-9913 kvanalstyne@winnipeg.ca
2014
September, 15th
An assessment of diagnostic performance of a filter-based antibody-independent peripheral blood circulating tumour cell capture paired with cytomorphologic criteria for the diagnosis of cancer.
An assessment of diagnostic performance of a filter-based antibody-independent peripheral blood circulating tumour cell capture paired with cytomorphologic criteria for the diagnosis of cancer.
Abstract
OBJECTIVES:An assessment of diagnostic performance of a filter-based antibody-independent peripheral blood circulating tumour cell capture paired with cytomorphologic criteria for the diagnosis of cancer
Circulating tumour cells (CTCs) are reported to be predictive for prognosis and response to treatment in advanced lung cancer. However, the clinical utility of the CTCs detection remains unknown for early stage lung cancer as the number of CTCs is reported as low, providing challenges in identification. We have evaluated diagnostic performance of filtration-based technology using cytomorphologic criteria in patients undergoing surgery for lung cancer.
MATERIAL AND METHODS:
We processed blood from 76 patients undergoing surgery for known or suspected lung cancer using ScreenCell(®) Cyto filter devices. Captured cells were stained using haematoxylin and eosin and independently assessed by two pathologists for the presence of atypical cells suspicious for cancer. Diagnostic performance was evaluated against pathologist reported diagnoses of cancer from surgically obtained specimens.
RESULTS:
Cancer was diagnosed in 57 patients (77.0%), including 32 with primary lung cancer (56.1%). The proportion of patients with early stage primary lung cancer in which CTCs were identified was 18 and 21 (56.3% and 65.6%, respectively) as reported by two pathologists. The agreement between the pathologists was 77.0% corresponding to a kappa-statistic of 53.7% indicating moderate agreement. No significant differences were found for the percentage of CTCs for primary and metastatic cancer as well as for cancer stages. On sensitivity weighted analysis, a sensitivity and specificity were 71.9% (95% CI 60.5-83.0) and 52.9% (95% CI 31.1-77.0), respectively. On specificity weighted analysis, a sensitivity and specificity were 50.9% (95% CI 39.3-64.4) and 82.4% (60.4-96.2), respectively.
The performance of the tested filter-based antibody-independent technology to capture CTCs using standard cytomorphologic criteria provides the potential of a diagnostic blood test for lung cancer.
June, 13th
Colorectal carcinomas in 2014: The search for powerful prognostic markers is still on the go!
Coget J, Borrini F, Susman S, Sabourin JC. Colorectal carcinomas in 2013: The search for powerful prognostic markers is still on the go! Cancer Biomark. 2014 Jan 1;14(2):145-50.
Abstract:
Colorectal cancer (CRC) is the third cause of cancer worldwide after prostate cancer and breast cancer. Patients have a survival rate of 5 years, which varies between 10 and 95% depending on the CRC stage.
Today, the management of patients with CRC is based on parameters such as TNM and classic histologic parameters, but new molecular and cell markers have been created to improve treatment and survival. Determining the expression of a characteristic set of genes either from formalin-fixed paraffin-embedded tissues (Onco type DX test™) or from fresh tissues (AGENDIA© ColoPrint®) has led to encouraging results, but there is a need for clinical validation on a large number of patients.
Also, next-generation sequencing (NGS) technologies may be the next step in the molecular approach of CRC tumor samples, allowing tumor characterization by gene signature arrays. In addition to molecular markers, evaluation of the presence of cellular markers such as circulating tumor cells (CTC) in the blood of patients with CRC can optimize prognostic evaluation and response to treatment. CTC isolation methods used today have different sensitivities and specificities, due not only to the very small number of these cells but also to the epithelial-mesenchymal transitional process (EMT). This paper presents the preliminary results of our study conducted on CTC isolation in patients with CRC by filtration method (Screencell Cyto). This fast and efficient method identifies CTCs and also isolates cells in EMT, which explains its high efficiency compared to technologies based on immunomagnetic and microfluidic separation reliant on EpCAM presence on the cell surface.
Cancer Biomarkers 2014 Colorectal carcinomas in 2013
April, 28th
Single Cell Analysis of Circulating Tumor Cells Identifies Cumulative Expression Patterns of EMT-Related Genes in Metastatic Prostate Cancer
Chen CL, Mahalingam D, Osmulski P, Jadhav RR, Wang CM, Leach RJ, Chang TC, Weitman SD, Kumar AP, Sun L, Gaczynska ME, Thompson IM, Huang TH.Single-cell analysis of circulating tumor cells identifies cumulative expression patterns of EMT-related genes in metastatic prostate cancer. Prostate. 2013 Jun; 73(8):813-26.
Abstract
BACKGROUND: Prostate tumors shed circulating tumor cells (CTCs) into the blood stream. Increased evidence shows that CTCs are often present in metastatic prostate cancer and can be alternative sources for disease profiling and prognostication. Here we postulate that CTCs expressing genes related to epithelial-mesenchymal transition (EMT) are strong predictors of metastatic prostate cancer.
METHODS: A microfiltration system was used to trap CTCs from peripheral blood based on size selection of large epithelial-like cellswithout CD45 leukocyte marker. These cells individually retrieved with a micromanipulator device were assessed for cell membrane physical properties using atomic force microscopy. Additionally, 38 CTCs from eight prostate cancer patients were used to determine expression profiles of 84 EMT-related and reference genes using a microfluidics-based PCR system.
RESULTS: Increased cell elasticity and membrane smoothness were found in CTCs compared to noncancerous cells, highlighting their potential invasiveness and mobility in the peripheral circulation. Despite heterogeneous expression patterns of individual CTCs, genesthat promote mesenchymal transitioning into a more malignant state, including IGF1, IGF2, EGFR, FOXP3, and TGFB3, were commonly observed in these cells. An additional subset of EMT-related genes (e.g., PTPRN2, ALDH1, ESR2, and WNT5A) were expressed in CTCs of castration-resistant cancer, but less frequently in castration-sensitive cancer.
CONCLUSIONS: The study suggests that an incremental expression of EMT-related genes in CTCs is associated with metastaticcastration-resistant cancer. Although CTCs represent a group of highly heterogeneous cells, their unique EMT-related gene signatures provide a new opportunity for personalized treatments with targeted inhibitors in advanced prostate cancer patients.
Copyright © 2012 Wiley Periodicals, Inc.
April, 28th
Rapid Separation of Mononuclear Hodgkin from Multinuclear Reed-Sternberg Cells
Lab Hematol 2014 Rapid Separation of Mononuclear Hodgkin from Multinuclear Reed-Sternberg Cells
Kongruttanachok N, Cayre YE, Knecht H, Mai S. Rapid separation of mononuclear hodgkin from multinuclear reed-sternberg cells. Lab Hematol. 2014 Mar 1;20(1):2-6.
We describe a method to isolate small mononucleated Hodgkin (H) cells from multinucleated Reed Sternberg (RS) cells of Hodgkinlymphoma using the ScreenCell filter device. This filtration-based approach lends itself to future clinical applications in that it enables the separation of H and RS cells from lymph node biopsies, bone marrow aspirates, pleural effusions, and blood, including the isolation of monoclonal Hodgkin precursor cells from the blood.
April, 25th
3D nuclear telomeric signatures define circulating tumor cells (CTCs) and characterize CTC subpopulations in intermediate risk prostate cancer patients
Awe, et.al. AACR April2014_pdf
2013September, 19th
Are morphological criteria sufficient for the identification of circulating tumor cells in renal cancer ?
El-Heliebi A, Kroneis T, Zöhrer E, Haybaeck J, Fischereder K, Kampel-Kettner K, Zigeuner R, Pock H, Riedl R, Stauber R, Geigl JB, Huppertz B, Sedlmayr P, Lackner C. Are morphological criteria sufficient for the identification of circulating tumor cells in renal cancer? J Transl Med. 2013 Sep 17; 11(1):214.
Abstract
BACKGROUND: Single circulating tumor cells (CTCs) or circulating tumor microemboli (CTMs) are potential biomarkers of renal cell cancer (RCC), however studies of CTCs/CTMs in RCC are limited. In this pilot study we aimed to evaluate a novel blood filtration technique suited for cytomorphological classification, immunocytochemical and molecular characterization of filtered, so called circulatingnon-hematologic cells (CNHCs) – putative CTCs/CTMs – in patients with RCC.
METHODS: Blood of 40 patients with renal tumors was subjected to ScreenCell filtration. CNHCs were classified according to cytomorphological criteria. Immunocytochemical analysis was performed with antibodies against CD45, CD31 and carbonic anhydrase IX (CAIX, a RCC marker). DNA of selected CNHCs and respective primary tumors was analysed by array-CGH.
RESULTS: CNHC-clusters with malignant or uncertain malignant cytomorphological features – putative CTMs – were negative for CD45, positive for CD31, while only 6% were CAIX positive. Array-CGH revealed that 83% of malignant and uncertain malignant cells did represent with a balanced genome whereas 17% presented genomic DNA imbalances which did not match the aberrations of the primary tumors. Putative single CTCs were negative for CD45, 33% were positive for CD31 and 56% were positive for CAIX.
CONCLUSIONS: The majority of CNHC-clusters, putative CTMs, retrieved by ScreenCell filtration may be of endothelial origin. Morphological criteria seem to be insufficient to distinguish malignant from non-malignant cells in renal cancer.
September, 19th
Detection of circulating tumor cells in patients with adrenocortical carcinoma: a monocentric preliminary study
Pinzani P, Scatena C, Salvianti F, Corsini E, Canu L, Poli G, Paglierani M, Piccini V, Pazzagli M, Nesi G, Mannelli M, Luconi M. Detection of circulating tumor cells in patients with adrenocortical carcinoma: a monocentric preliminary study. J Clin Endocrinol Metab. 2013 Sep; 98(9):3731-8.
Abstract:
CONTEXT: Adrenocortical carcinoma (ACC) is a rare malignancy, the prognosis of which is mainly dependent on stage at diagnosis. The identification of disease-associated markers for early diagnosis and drug monitoring is mandatory. Circulating tumor cells (CTCs) are released into the bloodstream from primary tumor/metastasis. CTC detection in blood samples may have enormous potential for assisting in the diagnosis of malignancy, estimating prognosis, and monitoring the disease.
OBJECTIVE: The aim of the study was to investigate the presence of CTCs in blood samples of patients with ACC or benign adrenocortical adenoma (ACA).
INTERVENTION: CTC analysis was performed in blood samples from 14 ACC patients and 10 ACA patients. CTCs were isolated on the basis of cell size by filtration through ScreenCell devices, followed by identification according to validated morphometric criteria and immunocytochemistry. We measured the difference in CTC detection between ACC and ACA.
RESULTS: CTCs were detected in all ACC samples, but not in ACA samples. Immunocytochemistry confirmed the adrenocortical origin. When ACC patients were stratified according to the median value of tumor diameter and metastatic condition, a statistically significant difference was found in the number of CTCs detected after surgery. A significant correlation between the number of CTCs in postsurgical samples and clinical parameters was found for tumor diameter alone.
CONCLUSIONS: Our findings provide the first evidence for adrenocortical tumors that CTCs may represent a useful marker to support differential diagnosis between ACC and ACA. The correlation with some clinical parameters suggests a possible relevance of CTC analysis for prognosis and noninvasive monitoring of disease progression and drug response.
February, 19th
Three-Dimensional Telomeric Analysis of Isolated Circulating Tumor Cells (CTCs) Defines CTC Subpopulations.
Adebayo Awe J, Xu MC, Wechsler J, Benali-Furet N, Cayre YE, Saranchuk J, Drachenberg D, Mai S. Three-Dimensional Telomeric Analysis of Isolated Circulating Tumor Cells (CTCs) Defines CTC Subpopulations. Transl Oncol. 2013 Feb; 6(1):51-65.
January, 19th
Usefulness of circulating tumor cell detection in pancreatic adenocarcinoma diagnosis
Iwanicki-Caron I, Basile P, Toure E, Antonietti M, Lecleire S, Di Fiore A, Oden-Gangloff A, Blanchard F, Lemoine F, Di Fiore F, Sabourin JC, Michel P. Usefulness of circulating tumor cell detection in pancreatic adenocarcinoma diagnosis. Am J Gastroenterol. 2013 Jan;108 (1):152-5.
2011
February, 19th
A new device for rapid isolation by size and characterization of rare circulating tumor cells.
Desitter I, Guerrouahen BS, Benali-Furet N, Wechsler J, Jänne PA, Kuang Y, Yanagita M, Wang L, Berkowitz JA, Distel RJ, Cayre YE. A new device for rapid isolation by size and characterization of rare circulating tumor cells. Anticancer Res. 2011 Feb; 31(2):427-41.