| 产品名称 | Multiaxial Mechanical Testing of Tissues and Biomaterials |
| 品牌 | |
| 产品货号 | Multiaxial Mechanical Testing of Tissues and Biomaterials |
| 产品价格 | 现货询价 |
| 联系人 | Mr. li |
| 联系电话 | 0086-18618101725 |
| 产品说明 Multiaxial Mechanical Testing of Tissues and Biomaterials
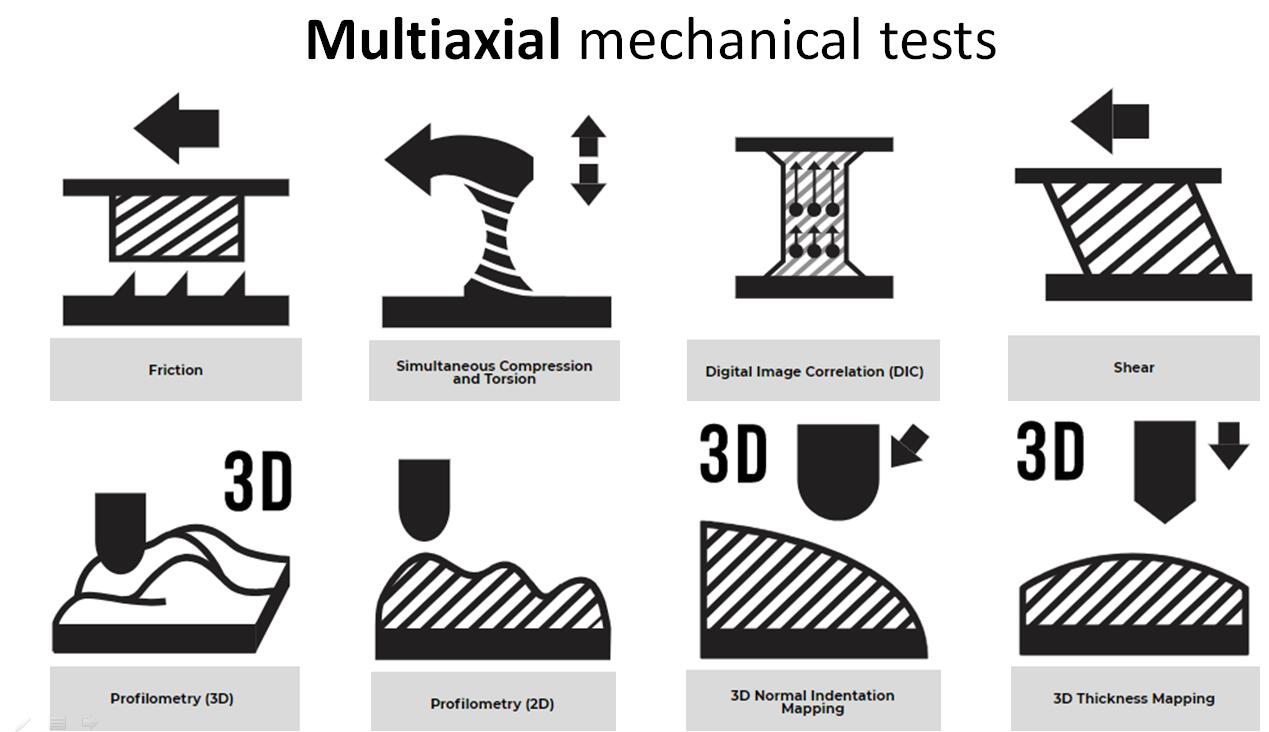
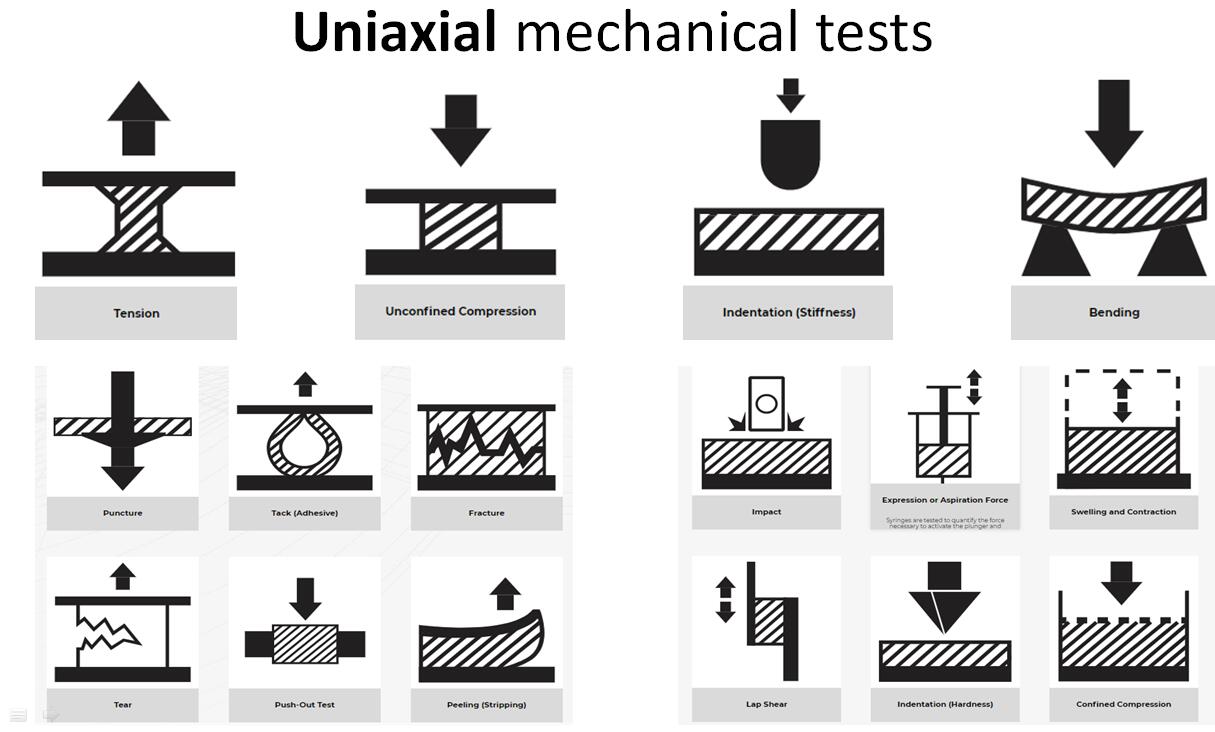
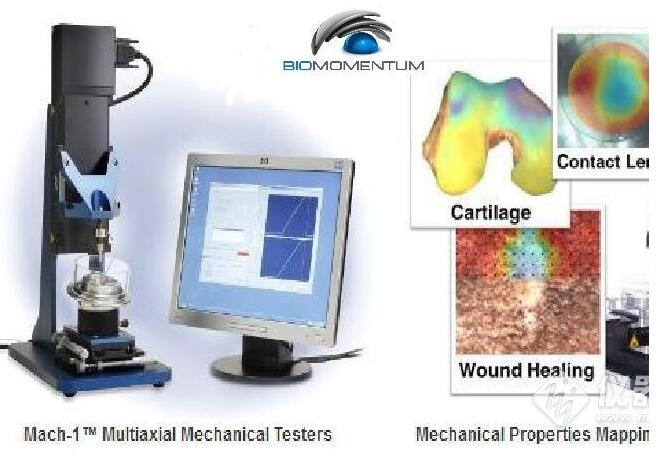
The Mach-1? multiaxial mechanical tester is the only all-in-one device designed for compression, tension, shear, friction, torsion and indentation mapping. The
Mach-1? is used in many university labs and is deemed an excellent educational tool。
please email slby800@163.com Or call 0086-18618101725
Since 1999, our unique multiaxial mechanical tester has helped hundreds of scientists around the world to enhance and publish their innovative research actives related to biomaterials, tissues and soft materials.
The MachOne
TM multiaxial mechanical tester is the only all-in-one device designed for compression, tension, shear, friction, torsion and indentation mapping.
1 AUTOMATED INDENTATION MAPPING 2 AUTOMATED THICKNESS MAPPING OF AN ARTICULAR SURFACE 3 U N C O N F I N E D C O M P R E S S I O N O F A N A R T I C U L A R C A R T I L A G E O S T E O C H O N D R A L C O R E 4 DYNAMIC MECHANICAL TESTING 6 UNCONFINED COMPRESSION OF A DISK 7 CONFINED COMPRESSION OF A CARTILAGE DISK
8 SHEAR TESTING ON A CARTILAGE DISK OR AN OSTEOCHON DRAL CORE 10 F R I C T I O N T E S T I N G O N A C A R T I L A G E D I S K O R A N O S T E O C H O N D R A L C O R E 11 E X T R A C T I O N A N D P R E P A R A T I O N O F C A R T I L A G E D I S K S A N D O S T E O C H O N D R A L C O R E S F R O M A N A R T I C U L A R
S U R F A C E
12 EXTRACTION OF ARTICULAR SURFACES FROM A CLOSED STIFLE JOINT IN LARGE ANIMAL SPECIES
13 3-POINT OR 4- POINT BENDING 14 Lap Shear Testing 15 C H A R A C T E R I Z A T I O N O F T H E M E C H A N I CA L P R O P E R T I E S O F P H A R M A C E U T I C A L T A B L E T S
TESTING SERVICES
is a service provider of high quality mechanical testing on biomaterials and tissues. Our worldwide clientele ranges from small medical device companies to well-established pharmaceutical firms. As experts in the field of biomedical engineering, we serve a multitude of industries while respecting the strict regulations that govern each business sector. offers a full-service approach to biomechanical testing. In addon to performing highly controlled tests using our state-of-the-art technology, our expert team adheres to effective standard operating procedures, develops reliable testing protocols, and delivers accurate data analysis reports in compliance with Good Laboratory Practice. As an extension of our services, we design and manufacture accessories tailored to meet our clients' specific study requirements. We collaborate with our clients and third-party CRO's to provide personalized testing solutions within a short time frame.
MECHANICAL TEST SERVICESWe offer a wide variety of mechanical tests for biomaterials, biological tissues and biomedical products. You might think your test idea is complex and hard to perform - we encourage you to share your idea with our study director. Every study is unique and we customize our services to match your specific needs - we adapt and perform different tests, different samples and different analysis for every client and every study. We, sometimes design new SOPs and protocols along with our clients, design and develop new accessories to perform different test types than what we usually do, perform different analysis than what we're used to - we're flexible! Please visit our Case Studies section to see a few examples of studies conducted by our team.
STUDY MANAGEMENTcan manage entire pre-clinical studies. Contact us to discuss our turn-key solutions to your pre-clinical study challenges.
From the early study design, to protocol development, overseeing proper conduct of the study, statistical analysis, quality assurance, we could manage your entire study to help you achieve your research objectives.
QUALITY MANAGEMENT SYSTEMWe recognize our responsibility as a provider of medical devices, mechanical testers and laboratory testing services to the most stringent quality standards. To this end, we have developed and documented a strong Quality Management System which provides evidence of strict quality controls implemented to ensure product and service quality. It complies with ISO 13485:2016 and EN ISO 13485:2016 (Medical Devices), with the FDA 21 CFR Part 820, with the European MDD 93/42/EEC and with the Canadian Medical Device Regulations 1998-783.
OTHER LAB ANALYSESworks with a network of qualified subcontractors providing a wide selection of high-quality addonal analytic results to what their scientists will offer you. Sample collection and handling, test protocols, quality assurance, data analysis in the context of a study are managed by and included in your final report.
Examples of addonal lab analysis offered: histology, uCT, blood works, etc.
GLP COMPLIANCE STATISTICAL ANALYSISOur scientists will assist you in the development of statistically-sound studies. We are committed to help you minimizing the number of animals involved in your studies, the number of measurements as well as related costs; while making sure that, at the end, you will be able to support study's conclusions with the necessary statistical power. When insufficient preliminary data are available to determine the optimal sample size, we will suggest to run short a pilot study to maximize your chance of success. | |