水凝支架三维培养系统,水凝胶基质力学环境模拟系统,水凝胶基质力学环境系统,生物材料细胞力学微环境体外构建系统
型号:fx-5000tt
联系人:李胜亮
联系电话:18618101725
品牌:美国flexercell
三维种子细胞构建人工生物组织系统(Creating a Bioartifical Construct with the Tissue Train System)
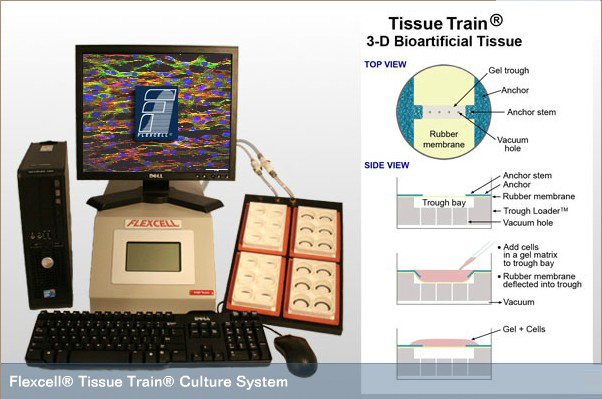 |
特点:
1)对生长在三维状态下的细胞进行静态的或者周期性的牵张拉伸刺激培养,可以进行实时观察分析。
2)对生长在三维环境下的细胞进行单轴向或者双轴向的静态或者周期性的应力加载实验
3)可建立te制的各种模拟实验:心率模拟实验,步行模拟实验,跑动模拟实验和其他动力模拟实验。
4)可构建长度达35mm的生物人工组织
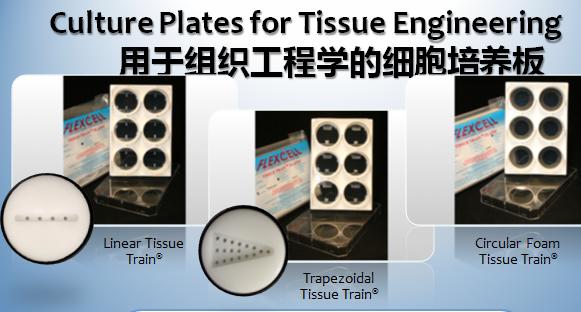
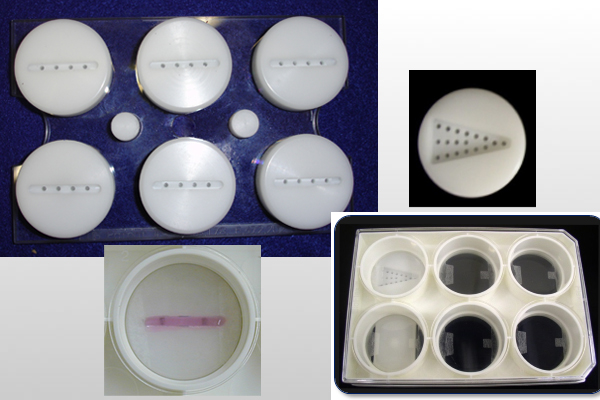
5)具有丰富的三维培养模具和多种蛋白包被材料的自动细胞组织三维培养系统
6)该系统以立体水凝胶为三维培养支架, 水凝胶支架具有大量体内微环境基质的特征,水凝胶所具有的三维网络结构、含水量高和力学性能可控等特性与体内细胞所处基质微环境相似, 被广泛用于工程化组织的体外构建研究,水凝胶的硬度调控范围很大, 非常有利于模拟体内生理或病理力学微环境
是真正意义上的三维培养系统

7)配套的scanflex扫描分析模块可以记录三维人工组织中凝胶的压实过程、记录三维细胞培养凝胶的压实动力学、凝胶面积计算
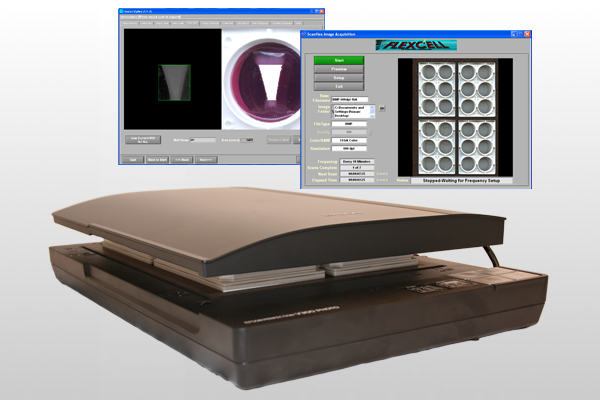
An automated scanning device with area measurement software.
- Measure gel compaction in 3D bioartificial tissues.
- Scans and saves images up to 600 dpi of 3D tissue constructs.
- Can be used in conjunction with the Tissue Train® Culture System.
适用范围
1)flexercell的Tissue Train ®培养体系,是为了解决这一组织培养过程中的难题,这个培养体系通过为细胞和基质提供三维支架矩阵组织、动态的拉伸力和多种几何模型来创建不同形状的生物人工组织(如线性,梯形和圆形)。
2水凝胶基质力学环境模拟
3)生物材料的细胞力学微环境体外构建系统
4)基于干细胞3D力学环境的工程化微组织构建研究
A 3D collagen cell-seeded construct (or bioartifical tissue) is dispensed with a pipette into a linear mold created with the
Trough Loader® and Tissue Train® System. After the construct has polymerized, the flexercell® Tension System can be used with an Arctangle® Loading Station? to apply uniaxial strain to the construct.
Tissue Train® Bioartificial Tissue Fabrication with Uniaxial Strain
Tension Test of a Bioartificial Tissue
A 3D cell-seeded collagen gel created with the Tissue Train® System is subjected to a tensile test until failure. Shown here is the construct within the test grips during testing
Tissue Train® Trapezoidal Construct under Tension with Corresponding Finite Element Strain Values
Trapezoidal-shaped 3D cell-seeded gel construct (created with the flexercell® Tissue Train®System) undergoing unconstrained tension applied with the FX-5000? Tension System. The strain values, as determined with Finite Element Analysis, are depicted alongside the strained construct.
Tissue Train ®培养系统应用背景
体外培养在与真实组织在结构上和功能上相似的人工组织需要以下几个基本条件:
(1)细胞
(2)支架矩阵组织
(3)培养基和生长因子和(4)机械刺激。这些条件彼此相互影响,并且相互之间共同来促进形成能够承受生物机械力的,且结构比较稳定的组织。而在人工组成形成的过程中,这些细胞按照发育途径形成具有一定几何形状的细胞外基质结构。其中一些信号转导途径参与了细胞外基质组合物的形成。这些途径中,有些是由细胞基质的机械变形调节,并通过膜结合蛋白,如整合素,粘着斑复合体,细胞粘附分子和离子通道传递到细胞内。这些途径中细胞还可以响应配体,如细胞基质形变所释放的细胞因子,激素或生长因子等。
为了维持肌肉骨骼组织的完整性和强度,组织内细胞需要保持一定水平的的内在应力。如果缺乏这种内在的应力,组织会缺少强度导致细胞结构的破坏或者组织的断裂。目前一般认为如果在固定四肢,卧床休息或在内在应力水平的降低的情况下,将导致骨中矿物质流失,骨组织萎缩,骨骼弱化,以及合成代谢活性的降低和分解代谢活性的增加。
为了在体外培养与原生组织类似的人工组织,重要的就是能够创建模拟体内条件的环境。细胞在具有机械运动作用的的环境中培养,可以促进细胞的新陈代谢,并可以改变细胞的形状和其它性能。因此,在体外形成过程中建立和保持一个具备机械作用的环境(即张力,剪切力或压缩)就成为这一过程中至关重要的。除了具备机械作用的环境,在三维环境下培养细胞可以比静态二维培养法更好地模拟原生环境。


典型应用文献摘选:
1. Abraham T, Kayra D, McManus B, Scott A. Quantitative
assessment of forward and backward second harmonic three dimensional images of
collagen type I matrix remodeling in a stimulated cellular environment. J
Struct Biol 180(1):17-25, 2012.
2. Ahearne M, Bagnaninchi PO, Yang Y, El Haj AJ. Online
monitoring of collagen fibre alignment in tissue-engineered tendon by PSOCT. J
Tissue Eng Regen Med 2(8):521-524, 2008.
3. Allison DA, Wight TN, Ripp NJ, Braun KR, Grande-Allen KJ. Endogenous
overexpression of hyaluronan synthases within dynamically cultured collagen
gels: implications for vascular and valvular disease. Biomaterials 29:2969-2976,
2008.
4. Barbolina MV, Liu Y, Gurler H, Kim M, Kajdacsy-Balla AA, Rooper
L, Shepard J, Weiss M, Shea LD, Penzes P, Ravosa MJ, Stack MS. Matrix
rigidity activates Wnt signaling through down-regulation of Dickkopf-1 protein.
J Biol Chem 288(1):141-51, 2013.
5. Bertrand AT, Ziaei S, Ehret C, Duchemin H, Mamchaoui K, Bigot A,
Mayer M, Quijano-Roy S, Desguerre I, Lainé J, Ben Yaou R, Bonne G, Coirault C. Cellular
microenvironments reveal defective mechanosensing responses and elevated YAP
signaling in LMNA-mutated muscle precursors. J Cell Sci 127(Pt
13):2873-84, 2014.
6. Cao TV, Hicks
MR, Campbell D, Standley PR. Dosed myofascial release in three-dimensional
bioengineered tendons: effects on human fibroblast hyperplasia, hypertrophy,
and cytokine secretion. J Manipulative Physiol Ther 36(8):513-21, 2013.
7. Cao TV, Hicks MR, Zein-Hammoud M, Standley PR. Duration
and magnitude of myofascial release in 3-dimensional bioengineered tendons:
effects on wound healing. J Am Osteopath Assoc 115(2):72-82, 2015.
8. Charoenpanich A, Wall ME, Tucker CJ, Andrews DM,
Lalush DS, Loboa EG. Microarray analysis of human adipose-derived stem
cells in three-dimensional collagen culture: osteogenesis inhibits bone
morphogenic protein and Wnt signaling pathways, and cyclic tensile strain
causes upregulation of proinflammatory cytokine regulators and angiogenic
factors. Tissue Eng Part A 17(21-22):2615-2627, 2011.
9. Clause KC, Tinney JP, Liu LJ, Gharaibeh B, Huard
J, Kirk JA, Shroff SG, Fujimoto KL, Wagner WR, Ralphe JC, Keller BB, Tobita K. A
three-dimensional gel bioreactor for assessment of cardiomyocyte induction in
letal muscle-derived stem cells. Tissue Eng Part C Methods 16(3):375-385,
2010.
10. Clause KC, Tinney JP, Liu LJ, Keller BB, Tobita
K. Engineered early embryonic cardiac tissue increases cardiomyocyte proliferation
by cyclic mechanical stretch via p38-MAP kinase phosphorylation. Tissue
Engineering Part A 15(6):1373-1380, 2009.
11. Clause KC, Tinney JP, Liu JL, Keller BB, Huard
J, Tobita K. p38MAP-kinase regulates cardiomyocyte proliferation and
contractile properties of engineered early embryonic cardiac tissue [abstract]. Weinstein Cardiovascular Development Research Conference, Indianapolis,
IN, 2007.
12. Clause KC, Tinney JP, Liu JL, Gharaibeh B,
Fujimoto LK, Wagner WR, Ralphe JC, Keller BB, Huard J, Tobita K. Functioning
engineered cardiac tissue from letal muscle derived stem cells [abstract]. 4th Annual Symposium of AHA
Council on Basic Cardiovascular Sciences, Keystone CO, 2007.
13. de Jonge N, Foolen J, Brugmans MC, S?ntjens SH,
Baaijens FP, Bouten CV. Degree of scaffold degradation influences collagen
(re)orientation in engineered tissues. Tissue Eng Part A 20(11-12):1747-57,
2014.
14. de Lange WJ, Grimes AC, Hegge LF, Ralphe JC. Ablation
of cardiac myosin-binding protein-C accelerates contractile kinetics in
engineered cardiac tissue. J Gen Physiol 141(1):73-84, 2013.
15. Ferdous Z, Lazaro LD, Iozzo RV, H??k M,
Grande-Allen KJ. Influence of cyclic strain and decorin deficiency on 3D
cellularized collagen matrices. Biomaterials 29(18):2740-2748, 2008.
16. Freeman SA, Christian S, Austin P, Iu I, Graves
ML, Huang L, Tang S, Coombs D, Gold MR, Rolley CD. Applied stretch
initiates directional invasion through the action of Rap1 GTPase as a tension
sensor. J Cell Sci 130(1):152-163, 2017.
17. Garvin J, Qi J, Maloney M, Banes AJ. Novel
system for engineering bioartificial tendons and application of mechanical
load. Tissue Eng 9(5):967-979, 2003.
18. Henshaw DR, Attia E, Bhargava M, Hannafin JA. Canine
ACL fibroblast integrin expression and cell alignment in response to cyclic
tensile strain in three-dimensional collagen gels. J Orthop Res 24(3):481-490,
2006.
19. Huang G, Wang L, Wang S, Han Y, Wu J, Zhang Q,
Xu F, Lu TJ. Engineering three-dimensional cell mechanical microenvironment
with hydrogels. Biofabrication 4(4):042001, 2012.
20. Jobling AI, Gentle A, Metlapally R, McGowan BJ,
McBrien NA. Regulation of scleral cell contraction by transforming growth
factor-? and stress: competing roles in myopic eye growth. J Biol Chem 284(4):2072-2079,
2009.
21. Jones ER, Jones GC, Legerlotz K, Riley GP. Cyclical
strain modulates metalloprotease and matrix gene expression in human tenocytes
via activation of TGFβ. Biochim Biophys Acta 1833(12):2596-2607, 2013.
22. Lee CH, Shin HJ, Cho IH, Kang YM, Kim IA, Park
KD, Shin JW. Nanofiber alignment and direction of mechanical strain affect
the ECM production of human ACL fibroblast. Biomaterials 26(11):1261-1270,
2005.
23. Masumoto H, Nakane T, Tinney JP, Yuan F, Ye F,
Kowalski WJ, Minakata K, Sakata R, Yamashita JK, Keller BB. The myocardial
regenerative potential of three-dimensional engineered cardiac tissues composed
of multiple human iPS cell-derived cardiovascular cell lineages. Sci Rep 6:29933,
2016.
24. Nguyen MD, Tinney JP, Ye F, Elnakib AA, Yuan F,
El-Baz A, Sethu P, Keller BB, Giridharan GA. Effects of physiologic
mechanical stimulation on embryonic chick cardiomyocytes using a microfluidic
cardiac cell culture model. Anal Chem 87(4):2107-13, 2015.
25. Nieponice A, Maul TM, Cumer JM, Soletti L, Vorp
DA. Mechanical stimulation induces morphological and phenotypic changes in
bone marrow-derived progenitor cells within a three-dimensional fibrin matrix. J
Biomed Mater Res A 81(3):523-530, 2007.
26. Nourse MB, Halpin DE, Scatena M, Mortisen DJ,
Tulloch NL, Hauch KD, Torok-Storb B, Ratner BD, Pabon L, Murry CE. VEGF
induces differentiation of functional endothelium from human embryonic stem
cells: implications for tissue engineering. Arterioscler Thromb Vasc Biol 30(1):80-89,
2010.
27. Peters AS, Brunner G, Krieg T, Eckes B. Cyclic mechanical strain induces
TGFβ1-signalling in dermal fibroblasts embedded in a 3D collagen lattice. Arch
Dermatol Res 307(2):191-7, 2015.
28. Qi J, Chi L, Bynum D, Banes AJ. Gap junctions
in IL-1β-mediated cell survival response to strain. J Appl Physiol 110(5):1425-1431,
2011.
29. Qi J, Chi L, Faber J, Koller B, Banes AJ. ATP
reduces gel compaction in osteoblast-populated collagen gels. J Appl Physiol 102(3):1152-60, 2007.
30. Qi J, Chi L, Maloney M, Yang X, Bynum D, Banes
AJ. Interleukin-1? increases elasticity of human bioartificial tendons. Tissue
Eng 12(10):2913-2925, 2006.
31. Qi J, Fox AM, Alexopoulos LG, Chi L, Bynum D,
Guilak F, Banes AJ. IL-1??decreases the elastic modulus of human tenocytes. J Appl Physiol 101(1):189-95, 2006.
32. Qi J, Chi L, Wang J, Sumanasinghe R, Wall M,
Tsuzaki M, Banes AJ. Modulation of collagen gel compaction by extracellular
ATP is MAPK and NF-?B pathways dependent. Exp Cell Res 315(11):1990-2000,
2009.
33. Rathbone SR, Glossop JR, Gough JE, Cartmell SH. Cyclic tensile strain upon human mesenchymal stem cells in 2D and 3D
culture differentially influences CCNL2, WDR61 and BAHCC1 gene expression
levels. J Mech Behav Biomed Mater 11:82-91, 2012.
34. Raval KK, Tao R, White BE, De Lange WJ, Koonce
CH, Yu J, Kishnani PS, Thomson JA, Mosher DF, Ralphe JC, Kamp TJ. Pompe
disease results in a Golgi-based glycosylation deficit in human induced
pluripotent stem cell-derived cardiomyocytes. J Biol Chem 290(5):3121-36,
2015.
35. Ruan JL, Tulloch NL, Saiget M, Paige SL,
Razumova MV, Regnier M, Tung KC, Keller G, Pabon L, Reinecke H, Murry CE. Mechanical
stress promotes maturation of human myocardium from pluripotent stem
cell-derived progenitors. Stem Cells 33(7):2148-57, 2015.
36. Schmidt JB, Chen K, Tranquillo RT. Effects
of intermittent and incremental cyclic stretch on ERK signaling and collagen
production in engineered tissue. Cellular and Molecular Bioengineering 1-10,
2015.
37. Sumanasinghe RD, Bernacki SH, Loboa EG. Osteogenic
differentiation of human mesenchymal stem cells in collagen matrices: effect of
uniaxial cyclic tensile strain on bone morphogenetic protein (BMP-2) mRNA
expression. Tissue Eng 12(12):3459-3465, 2006.
38. Taylor SE, Vaughan-Thomas A, Clements DN,
Pinchbeck G, Macrory LC, Smith RK, Clegg PD. Gene expression markers of
tendon fibroblasts in normal and diseased tissue compared to monolayer and
three dimensional culture systems. BMC Musculolet Disord 10:27, 2009.
39. Tchao J, Han L, Lin B, Yang L, Tobita K. Combined
biophysical and soluble factor modulation induces cardiomyocyte differentiation
from human muscle derived stem cells. Sci Rep 4:6614, 2014.
40. Tchao J, Kim JJ, Lin B, Salama G, Lo CW, Yang
L, Tobita K. Engineered human muscle tissue from letal muscle derived
stem cells and induced pluripotent stem cell derived cardiac cells. Int J
Tissue Eng. 2013:198762, 2013.
41. Tobita K, Liu LJ, Janczewski AM, Tinney JP,
Nonemaker JM, Augustine S, Stolz DB, Shroff SG, Keller BB. Engineered early
embryonic cardiac tissue retains proliferative and contractile properties of
developing embryonic myocardium. Am J Physiol Heart Circ Physiol 291(4):H1829-37,
2006.
42. Tondon A, Haase C, Kaunas R. Mechanical
stretch assays in cell culture systems. In: Handbook of Imaging in
Biological Mechanics, ed. Neu CP, Genin GM. CRC Press: Boca Raton, 2015.
43. Triantafillopoulos IK, Banes AJ, Bowman KF Jr,
Maloney M, Garrett WE Jr, Karas SG. Nandrolone decanoate and load increase
remodeling and strength in human supraspinatus bioartificial tendons. Am J
Sports Med 32(4):934-943, 2004.
44. Tulloch NL, Muskheli V, Razumova MV, Korte FS,
Regnier M, Hauch KD, Pabon L, Reinecke H, Murry CE. Growth of engineered
human myocardium with mechanical loading and vascular coculture. Circ Res 109(1):47-59,
2011.
45. Weinbaum JS, Schmidt JB, Tranquillo RT.
Combating adaptation to cyclic stretching by prolonging activation of
extracellular signal-regulated kinase. Cellular and Molecular Bioengineering 6(3):279-286, 2013.
46. Wen W, Chau E, Jackson-Boeters L, Elliott C,
Daley TD, Hamilton DW. TGF-?1 and FAK regulate periostin expression in PDL
fibroblasts. J Dent Res 89(12):1439-1443, 2010.
47. Yang G, Rothrauff BB, Lin H, Gottardi R,
Alexander PG, Tuan RS. Enhancement of tenogenic differentiation of human
adipose stem cells by tendon-derived extracellular matrix. Biomaterials 34(37):9295-306,
2013.
48. Yang Y, Wimpenny I, Wang RK. Application of
polarization-sensitive OCT and Doppler OCT in tissue engineering. In: Optical
Techniques in Regnerative Medicine, edited by Morgan SP, Rose F, Matcher
SJ. Taylor & Francis Group: Florida, p. 307-327, 2014.
49. Ye F, Yuan F, Li X, Cooper N, Tinney JP, Keller BB. Gene expression
profiles in engineered cardiac tissues respond to mechanical loading and
inhibition of tyrosine kinases. Physiol Rep 1(5):e00078, 2013.
